Fecundation and Gravidity- Choriocarcinoma
Anubha Bajaj*
Histopathologist in A B Diagnostics, New Delhi, India
Submission: December 06, 2022; Published: January 02, 2023
*Corresponding Address: Anubha Bajaj, Histopathologist in A B Diagnostics, New Delhi, India
How to cite this article: Anubha B. Fecundation and Gravidity-Choriocarcinoma. Canc Therapy & Oncol Int J. 2023; 23(1): 556101. DOI:10.19080/CTOIJ.2023.23.556101
Mini Review
Choriocarcinoma is a malignant gestational trophoblastic neoplasm constituted of trilogy of syncytiotrophoblastic cells, cytotrophoblastic cells and intermediate trophoblastic cells. Tumefaction arises after preceding gestational events as complete hydatidiform mole and delineates aggressive biological behaviour. Serum beta human chorionic gonadotropin (β-hCG) is a reliable indicator of tumour occurrence and progression. Neoplasm can be alleviated with precise chemotherapy. Generally, choriocarcinoma incriminates females within reproductive age group. Choriocarcinoma common arises within uterus. Fallopian tubes or ovaries may be infrequently involved [1,2].
Choriocarcinoma emerges from trophoblastic cellular component of preceding gestation. Clinical history of preceding pregnancy, commonly a complete hydatidiform mole is elicited. Infrequently, choriocarcinoma arises following non molar abortion or ectopic gestation. Intra-placental choriocarcinoma may emerge after non-molar gestation and may be within third trimester or postpartum period. Choriocarcinoma frequently manifests with vaginal bleeding. However, neoplasm may initially represent with metastatic disease, especially when associated with tumefaction occurring after non-molar gestation [1,2].
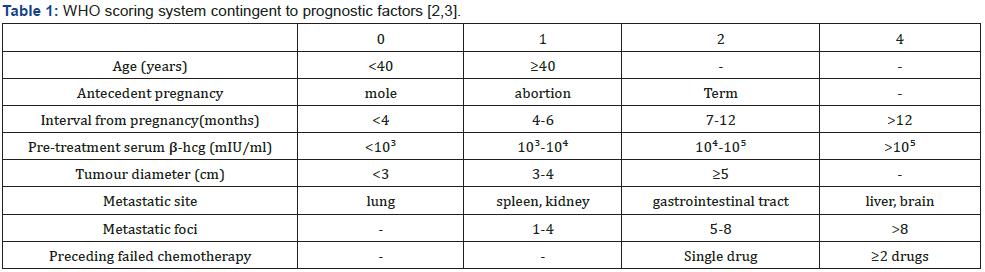
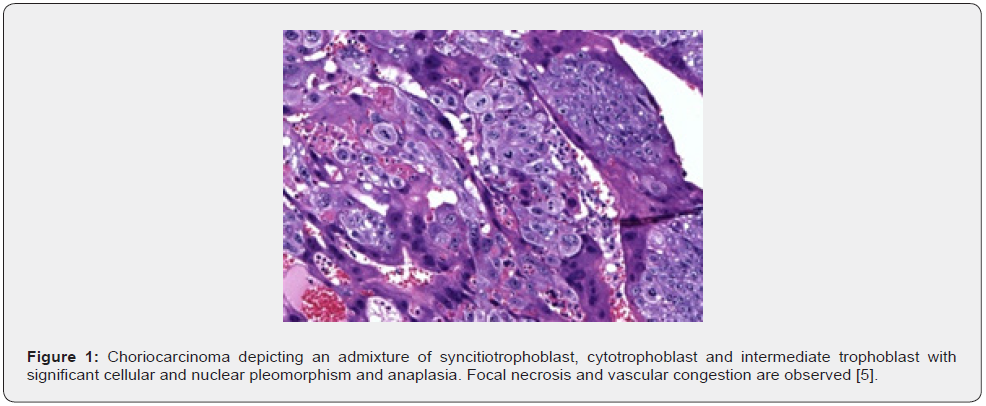
Tumour metastasis may ensue within pulmonary parenchyma associated with dyspnoea or haemoptysis, lower genital tract manifesting violet nodules situated upon vulva, vagina or cervix, hepatic parenchyma with abnormal liver function or intraabdominal haemorrhage and brain demonstrating neurological symptoms as convulsion or altered mental status [1,2]. Cytological examination is exceptionally performed and demonstrates clusters of cohesive, atypical epithelioid cells. Generally, dual cell population composed of enlarged cells imbued with abundant cytoplasm and intracellular globules appear admixed with miniature cells demonstrating hyperchromatic nuclei and elevated nucleo-cytoplasmic (N/C) ratio. Multinucleated cells can be occasionally discerned. Grossly, a dark red, solid, friable tumefaction is delineated. Tumefaction is commonly situated within endometrium or myometrium. Cut surface exhibits foci of haemorrhage and necrosis [1,2].
Macroscopically, intra-placental choriocarcinoma may manifest as a subtle lesion and may be misinterpreted as placental infarct. Upon microscopy, the infiltrative, destructive neoplasm configures solid sheets of atypical syncytiotrophoblastic cells and cytotrophoblastic cells intermingled with intermediate trophoblastic cells. Tumefaction is devoid of chorionic villi [1,2]. Nevertheless, an intra-placental choriocarcinoma may be circumscribed by chorionic villi. Mitotic activity is significant. Focal necrosis and haemorrhage are observed. Few neoplasms may represent as mixed trophoblastic tumour with a component of placental site trophoblastic tumour or epithelioid trophoblastic tumour [1,2].
Anatomic staging of choriocarcinoma as per International Federation of Obstetrics and Gynaecology (FIGO) is designated as
• stage I: Tumour confined to uterine corpus
• stage II: Tumour extending beyond uterus into uterine adnexa, vagina or various sites of female genital tract
• stage III: Tumour extending into pulmonary parenchyma along with or devoid of incrimination of female genital tract
• stage IV: Tumours metastases into diverse sites [2,3].
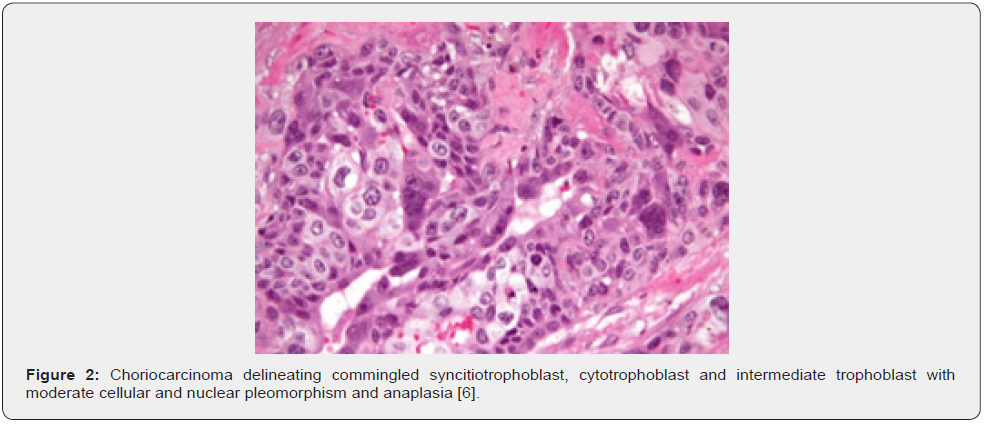
Prognostic outcomes of choriocarcinoma may be assessed with World Health Organization (WHO) prognostic scoring system, contingent to factors such as age, antecedent pregnancy, interval between diagnosis and index pregnancy, serum β-hCG levels, magnitude of largest tumour, site of quantifiable metastasis and resistance to pertinent chemotherapy [2,3]. Thus categorized, choriocarcinoma is subdivided into
• low risk: ≤ 6 numeric score
• high risk: ≥ 7 numeric score
• ultra high risk: > 12 numeric score [2,3].
TNM classification of choriocarcinoma is designated as
Primary tumour
• TX: Primary tumour cannot be assessed
• T0: No evidence of primary tumour
• T1: Tumour confined to uterus
• T2: Tumour extends to genital viscera as ovary, fallopian tube, vagina or broad ligaments as direct extension or distant metastasis.
Regional lymph nodes
Staging of gestational trophoblastic tumours is devoid of pertinent designation of regional lymph node metastasis. Incrimination of regional lymph nodes is categorized as metastatic disease (M1b).
Distant metastasis
• M0: Distant metastasis absent
• M1: Distant metastasis present ~M1a: Metastasis into pulmonary parenchyma ~M1b: Metastasis or direct invasion into distant, non-genital anatomic structures [2,3].
TNM descriptions for specific instances are denominated as
• ’m’: Multiple, primary tumours confined to a singular site
• ’y’: Tumour classification obtained during or following initial therapy
• ’r’: Recurrent tumour following documented disease-free interval
• ’a’: Tumour stage obtained at autopsy [2,3].
Residual tumour is categorized as
• Rx: Residual tumour cannot be assessed
• R0: Residual tumour absent
• R1: Microscopic persistence of residual tumour
• R2: Macroscopic determination of residual tumour [2,3].
Choriocarcinoma is immune reactive to AE1/ AE3 or cytokeratin7. Ki67 proliferation index is > 90%. Syncytiotrophoblast is immune reactive to HCG, inhibin or HSD3B. Cytotrophoblast is immune reactive to SALL4, nuclear β catenin or GATA3. Intermediate trophoblast is immune reactive to human placental lactogen (hPL), HLA-G, Mel-CAM(CD146), MUC4, inhibin, HSD3B1, GATA3 and focally immune reactive to p63. Choriocarcinoma is immune non-reactive to p16 [3,4]. The exceptionally performed ultrastructural examination exhibits a neoplasm with tri-phasic cellular population composed of syncytiotrophoblast, cytotrophoblast, and intermediate trophoblast. Zones of cellular transition between diverse cellular components can be abrupt. Transitional cellular configurations are absent [3,4]. Syncytiotrophoblast exemplifies complex neoplastic cells imbued with dense, abundant cytoplasm, multiple, irregular, convoluted nuclei along with distended endoplasmic reticulum, Golgi apparatus, lysosomes and tonofilaments. Syncytiotrophoblastic cell membrane enunciates innumerable microvilli. Cytotrophoblast exhibits adjacent epithelial cells permeated with simple cytoplasm incorporating minimal organelles, singular, enlarged nucleus and intracytoplasmic glycogen. Intermediate trophoblast is comprised of mononuclear cells of intermediate magnitude pervaded with moderately complex cytoplasm and several organelles [3,4].
Genotyping with short tandem repeat (STR) analysis can appropriately segregate gestational and non-gestational genesis of choriocarcinoma with documentation of paternal alleles discerned within gestational choriocarcinoma. Besides, the manoeuver can detect index pregnancy. Majority of choriocarcinomas depict XX sex chromosomes and complex karyotypes [3,4]. Choriocarcinoma requires segregation from neoplasms such as placental site trophoblastic tumour, epithelioid trophoblastic tumour, hydatidiform mole, invasive mole, early pregnancy loss, non-gestational choriocarcinoma, seminoma, mixed germ cell tumour, solid variant of yolk sac tumour or embryonal carcinoma [3,4].
Choriocarcinoma delineates significantly elevated serum β-hCG levels, which is a reliable indicator of neoplastic occurrence and tumour burden. Thus, serum β-hCG can be adopted for diagnosis, follow up, evaluation of response to therapy and screening for tumour reoccurrence [3,4]. Surgical tissue sampling is infrequently obtained for tumour diagnosis. Upon ultrasonography, pelvic tumefaction appears as a heterogeneous, hypoechoic or hyperechoic mass. Colour Doppler enunciates enhanced tumour vascularization with an extensively vascular tumour perimeter and avascular centric segment [3,4]. Plain radiograph of thoracic cavity is a preliminary imaging technique adopted to evaluate pulmonary metastases of choriocarcinoma. Computerized tomography (CT) and magnetic resonance imaging (MRI) can be employed to assess metastatic disease accompanying choriocarcinoma. MRI can be selectively employed to evaluate uterine and pelvic disease [3,4]. Typically, metastatic tumefaction manifests as multiple, well defined, peripheral, rounded, nodular lesions. Infrequently, pulmonary metastasis may manifest as a singular nodule, pulmonary cavitation, pleural effusion, atelectasis, or embolic disease [3,4].
Metastasis into hepatic parenchyma emerges as multiple, heterogeneous, rounded tumefaction. Upon CT, hepatic metastasis manifests as hypodense tumefaction. Following administration of intravenous contrast, enhancement within arterial phase is observed. Upon MRI, T2 weighted imaging of hepatic metastasis demonstrates hyper-intense tumefaction. Signal enhancement ensues following administration of gadolinium contrast [3,4]. Metastases into brain manifests as singular or multiple, heterogeneous tumour nodules, commonly situated upon interface of grey/white matter. CT exhibits enhanced tumour attenuation. MRI enunciates variable signal intensity [3,4].
Choriocarcinoma can be appropriately treated with chemotherapy, contingent to FIGO classification or WHO prognostic score. Low risk neoplasms can be subjected to singular chemotherapeutic agent as methotrexate or actinomycin D [3,4]. High risk neoplasms are treated with multiple chemotherapeutic agents as etoposide, methotrexate, actinomycin, cyclophosphamide, vincristine (EMA-CO) or etoposide, cisplatin - etoposide, methotrexate, actinomycin (EP-EMA) or paclitaxel, etoposide - paclitaxel, cisplatin (TE / TP) combination [3,4]. Surgical eradication is reserved for and recommended within pertinent instances as incriminated elderly subjects, resistance to chemotherapy, uterine rupture due to neoplastic occurrence or life-threatening haemorrhage [3,4].
Employment of radiotherapy is debatable. Radiotherapy can be adopted for treating metastasis to brain.
Inferior prognostic outcomes are observed with ~incriminated elderly subjects ~tumour occurrence within non molar pregnancy ~extended interval between index pregnancy and tumour diagnosis ~significantly elevated serum β-hCG levels ~significant tumour burden ~tumour metastasis into brain or hepatic parenchyma ~tumour resistance to chemotherapy [3,4].
References
- Bishop BN, Edemekong PF (2022) Choriocarcinoma. Stat Pearls International 2022, Treasure Island, Florida, USA.
- Katsanevakis E, Oatham A, Darly Mathew (2022) Choriocarcinoma After Full-Term Pregnancy: A Case Report and Review of the Literature. Cureus 14(2): e22200.
- Wang Y, Wang Z, Xiaoxu Zhu, Qihong Wan, Peilin Han, et al. (2022) Intestinal metastasis from choriocarcinoma: a case series and literature review. World J Surg Oncol 20(1): 173.
- Kazemi NY, Langstraat C, S John Weroha (2022) Non-gestational choriocarcinoma with hyperprogression on pembrolizumab: A case report and review of the literature. Gynecol Oncol Rep 39: 100923.
- Image 1 Courtesy: Pathology outlines
- Image 2 Courtesy: Wikipedia






























