The Afflicted Assimilation-Coeliac Disease
Anubha Bajaj*
Histopathologist in A B Diagnostics, New Delhi, India
Submission: October 11, 2022; Published: November 16, 2022
*Corresponding Address: Anubha Bajaj, Histopathologist in A B Diagnostics, New Delhi, India
How to cite this article: Anubha B. The Afflicted Assimilation-Coeliac Disease. Canc Therapy & Oncol Int J. 2022; 22(5): 556096. DOI:10.19080/CTOIJ.2022.22.556096
Mini Review
Coeliac disease or gluten sensitive enteropathy is an immune mediated inflammatory disease of small intestine. The condition arises within genetically predisposed individuals and may be engendered due to sensitivity to prolamins as wheat (gliadin), barley (hordein), rye (secalin) and oats (avenin). Activated innate and adaptive immune response to prolamins characteristically induces inflammatory infiltration of lamina propria and mucosal epithelium by chronic inflammatory cells as lymphocytes in concurrence with villous atrophy, features which engender intestinal malabsorption.
Intraepithelial lymphocytosis within small bowel appearing secondary to gluten ingestion is associated with partial or total atrophy of intestinal villi and hyperplasia of intestinal crypts along with chronic inflammatory exudate confined to lamina propria [1,2]. Additionally designated as coeliac sprue, sprue, non-tropical sprue, gluten sensitive enteropathy or gluten induced enteropathy, coeliac disease is preponderantly encountered within small intestine. Histological confirmation within small intestinal surgical tissue samples along with cogent serology are a pre-requisite for appropriate disease discernment [1,2].
A female predominance is observed with male to female proportion of 1:1.85. Coeliac disease enunciates a bimodal disease distribution at ~12months and third decade to fourth decade. Mean age of disease discernment is 8.4 years [1,2]. Coeliac disease may concur with autoimmune diseases as dermatitis herpetiformis, type 1 diabetes mellitus, Hashimoto’s thyroiditis, Graves’ disease, idiopathic disorders as dilated cardiomyopathy, epilepsy, multiple sclerosis and chromosomal diseases as Down’s syndrome, Turner’s syndrome, or William syndrome [1,2].
HLA class II genes as HLA DQ2 and HLA DQ8 situated upon chromosome 6 may induce genetic susceptibility to coeliac disease. Implication of first-degree relatives is indicative of significant hereditary component. HLA molecules represented upon antigen presenting cells introduce laminins to CD4+ T lymphocytes with consequent activation. Gluten rich diet configures as an environmental trigger. Fragments of gluten peptides resistant to degradation are transported across intestinal epithelium through transcellular pathways [1,2].
Altered intestinal permeability may concur with release of cellular contents or enzyme tissue transglutaminase (tTG). CD71 (transferrin) receptor appears upregulated within active coeliac disease [1,2]. Secretion of interferon gamma (IFNγ) and interleukin 21 induces epithelial damage following activation of T lymphocytes by antigen presenting cells [1,2]. Innate immune response is initiated by intraepithelial lymphocytes (IELs), which expound NK receptor MHC class I related chains A and B along with HLE confined to epithelial cells with propagation of epithelial destruction [1,2]. IL15 upregulates NK receptors situated within cytotoxic intraepithelial lymphocytes and induces T cell receptor independent cellular demise. Refractory sprue is denominated by a process wherein disease persists despite circumvention of dietary laminin and intraepithelial lymphocytes (IELs) acquire a predominantly activated NK phenotype [1,2].
Refractory disease is categorized as
• Refractory coeliac disease type I demonstrating intraepithelial lymphocytes which express CD3, CD8 and TCRβ, akin to preliminary or active coeliac disease. Type I exhibits a superior prognosis upon initiation of immunosuppressive therapy [1,2].
• Refractory coeliac disease type II is devoid of CD8, CD4 and TCRαβ and enunciates an adverse prognosis. Intracellular CD3ε and clonal TCR genetic rearrangement may be delineated [1,2]. Coeliac disease may be engendered due to genetic factors as HLA DQ2 and HLA DQ8, ingestion of gluten rich diet or variable innate and adaptive immune responses [1,2]. Besides, decimated diversity of gastrointestinal tract microbiota and elevated Firmicutes/Bacteroidetes species appear to be implicated. Paediatric coeliac disease characteristically exhibits diarrhoea, anorexia, abdominal distension, or failure to thrive [1,2]. Incriminated older children enunciate diarrhoea, bloating, constipation, abdominal pain, or weight loss [1,2]. Incriminated adults exemplify malabsorption syndrome associated with chronic diarrhoea, weight loss and asthenia [1,2].
Extra-intestinal coeliac disease manifests with:
i. Iron deficiency anaemia with microcytic blood picture
ii. Macrocytic anaemia due to deficient vitamin B12
iii. Osteopenia or osteoporosis due to altered absorption of calcium and vitamin D3
iv. Growth retardation or defective tooth enamel, aphthous stomatitis and elevated serum transaminases ~dermatitis herpetiformis
v. Nonspecific symptoms as headache, paraesthesia, neuro-inflammation, anxiety, or depression symptoms concurrent with reproductive function as delayed menarche, amenorrhea, recurrent miscarriage, premature birth, early menopause in females and altered quantification and mobility of male spermatozoa [1,2].
Refractory coeliac disease (RCD) is delineated by persistent malabsorption and villous atrophy despite strict adherence to gluten free diet for minimally 6 months to 12 months in the absence of associated active coeliac disease or overtly malignant disorders [1,2]. Type I and type II variant of refractory coeliac disease enunciates severe complications as ulcerative jejunitis or enteropathy associated T cell lymphoma [1,2].
Upon gross examination, villous flattening or blunting of small gastrointestinal tract is observed. Severe disease is associated with ulceration [1,2]. Appropriate microscopic evaluation may be achieved with one or two wisps of tissue obtained from duodenal bulb and minimally four specimens from post-bulbar duodenum [1,2]. Upon microscopy, elementary mucosal lesions can be discerned. Coeliac disease exemplifying increased intraepithelial T lymphocytes (IELs) is denominated as: • borderline lesions with 25 - 29 IELs/100 enterocytes • pathological lymphocytosis with > 30 IELs/100 enterocytes [1,2]. Features such as decreased enterocyte height, flattening of enterocytes, intracytoplasmic vacuoles and reduction or absence of brush border may indicate coeliac disease [1,2]. Crypt hyperplasia is comprised of extension of regenerative epithelial hyperplasia within crypts associated with mucosal alterations and > one mitosis per crypt [1,2]. Villous atrophy enunciates decimated villous height or normal villous: crypt ratio at 3:1 followed by complete disappearance of villi. Assessment of villous hypertrophy requires appropriate orientation of surgical tissue samples [1,2] (Figures 1 & 2) (Table 1).
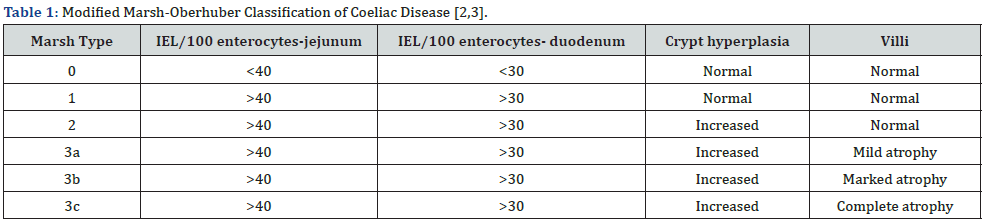
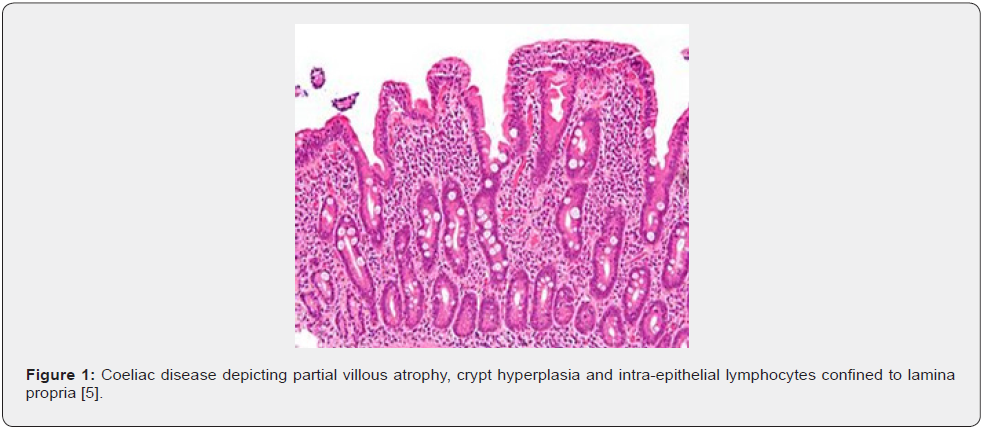
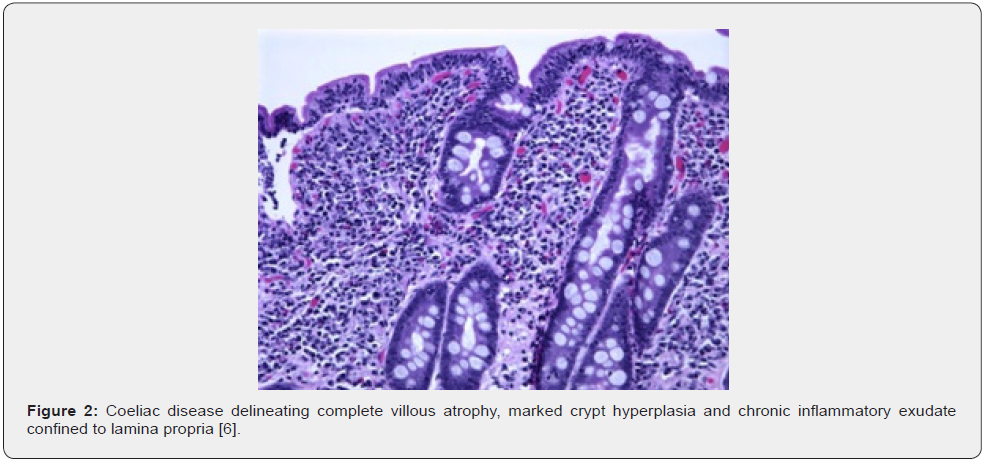
IEL/100 enterocytes: intraepithelial lymphocytes per 100 enterocytes,
Coeliac disease is categorized as:
• Type 0 exemplifying normal intestinal epithelium with improbable coeliac disease.
• Type I exhibiting coeliac disease associated with gluten free diet or minimal ingestion of gluten or gliadin, dermatitis herpetiformis, diverse infections, nonspecific factors, or family members of subjects with coeliac disease [2,3].
• Type II is exceptionally discerned and comprised of coeliac disease occasionally encountered with dermatitis herpetiformis.
• Type III elucidates spectrum of morphological alterations associated with symptomatic coeliac disease [2,3]. Grading of duodenal mucosal lesions of coeliac disease as per simplified system of Corazza, Roberts and Ensari is designated as
• Grade A/type I: increased intraepithelial lymphocytes in the absence of villous atrophy.
• Grade B1/type II: shortening and blunting of villi with symptomatic coeliac disease.
• Grade B2/type 3: complete villous atrophy with symptomatic coeliac disease [2,3].
Coeliac disease necessitates segregation from conditions such as tropical sprue, autoimmune enteropathy, common variable immunodeficiency, food allergy, intestinal malabsorption, Crohn’s disease, collagenous sprue, eosinophilic gastroenteritis, inflammatory bowel disease, HIV enteropathy, giardiasis, ingested drugs as sartans, mycophenolate or non-steroidal antiinflammatory drugs, intestinal lymphoma, bacterial or viral gastroenteritis or irritable bowel syndrome [3,4].
Pertinent investigative technique for ascertaining coeliac disease is cogent tissue sampling of duodenum with histological confirmation. Serologic assay is accomplished with evaluation of tTGA, EMA and IgA class antigliadin antibodies (AGA). Evaluation of serum tTGA is sensitive (98%) and specific (90%) for ascertaining coeliac disease although antibodies may be discerned within individuals upon gluten free diet [3,4]. Endoscopic evaluation of duodenum or small intestine is a pathognomonic investigative modality which exhibits patchy, duodenal villous atrophy, identifiable by magnification endoscopy or chromo-endoscopy. Also, features as scalloping or notching of mucosal folds can be observed [3,4]. HLA DQ association test is optimal for ascertaining HLA DQ2 and HLA DQ8 components, which may be discerned within populations uninvolved with coeliac disease [3,4]. Flow cytometry is optimally adopted for quantification of intraepithelial lymphocytes, designated as IEL lymphogram. Aforesaid graph demonstrates IELs as antigen experienced T lymphocytes which denominate αβ (> 90%) and γδ (< 10%) receptors [3,4].
Coeliac disease exhibits elevated total IELs along with permanently elevated γδ IELs and decimated CD3 IELs [3,4]. Refractory coeliac disease type II typically enunciates decimation of multiple surface T cell markers as CD3, CD7 or CD8 in > 20% of intraepithelial lymphocytes upon flow cytometry [3,4]. Upon fluoroscopy, features such as distension of small intestine, contrast dilution, multiple non-obstructing intussusceptions with a ‘coiled spring’ appearance, dilated jejunal loops with completely decimated jejunal folds or ‘moulage sign’ and flocculation on account of coarse clumps of disintegrated barium may be observed [3,4]. Upon computerized tomography or magnetic resonance imaging (CT / MRI), features such as jejunoileal fold pattern reversal, thickened ileal fold, perienteric stranding, regional lymph node enlargement, ascites or submucosal deposition of adipose tissue may be encountered, especially in disease of extensive duration [3,4]. Appropriate therapy of coeliac disease necessitates correction of nutritional deficiencies and circumvention of bone depletion. Vaccination for circumventing hepatitis B and pneumococcal infection is beneficial.
Gluten or laminin free diet is recommended [3,4]. Transglutaminase 2 inhibitors can be employed for treating coeliac disease. Dermatitis herpetiformis requires therapy with sulfones. Refractory coeliac disease type II appears resistant to gluten free diet or various treatment strategies [3,4]. Adoption of gluten free diet is associated with superior prognostic outcomes. Proportionate emergence of small bowel adenocarcinoma is elevated [3,4]. Severe complications as ulcerative jejunitis or enteropathy associated T cell lymphoma represent with an unfavourable prognosis [3,4]. Enteropathy associated T cell lymphoma may concur with refractory coeliac disease type II within five years of disease occurrence [3,4]. Secondary enteropathy associated T cell lymphoma may emerge in subjects with well controlled coeliac disease of extensive duration [3,4]. Enteropathy associated T cell lymphoma concurrent to coeliac disease or refractory coeliac disease manifests immune nonreactive CD56- along with gain of chromosomes 1q and 5q [3,4]. Classic subtype of enteropathy associated T cell lymphoma is frequently associated with coeliac disease [3,4].
References
- Posner EB, Haseeb M (2022) Celiac Disease. Stat Pearls International 2022, Treasure Island, Florida, USA.
- Gibson PR (2022) Coeliac disease in 2022. Aliment Pharmacol Ther 56 Suppl 1: S1-S2.
- Catassi C, Verdu EF, Julio Cesar Bai, Elena Lionetti (2022) Coeliac disease. Lancet 399(10344): 2413-2426.
- Durazzo M, Ferro A, Isabella Brascugli, Simone Mattivi , Sharmila Fagoonee, et al. (2022) Extra-Intestinal Manifestations of Celiac Disease: What Should We Know in 2022? J Clin Med 11(1): 258.
- Image 1 Courtesy: Libre Pathology.
- Image 2 Courtesy: Uptodate.com






























