Effect of Triple IV therapy (Artesunate, Ascorbate, and Doxycycline) on Circulating Tumor Cells and DNA: Two Consecutive Cases
Ksenia Lynch and Leanna Standish*
AIMS Institute, Seattle, WA, USA
Submission: October 17, 2022; Published: October 26, 2022
*Corresponding Address: Leanna Standish, AIMS Institute, Seattle, WA, USA
How to cite this article: Ksenia L, Leanna S. Effect of Triple IV therapy (Artesunate, Ascorbate, and Doxycycline) on Circulating Tumor Cells and DNA: Two Consecutive Cases . Canc Therapy & Oncol Int J. 2022; 22(4): 556092. DOI:10.19080/CTOIJ.2022.22.556092
Background
Artesunate (ART) is synthesized from artemisinin, an extract from the sweet wormwood plant, Artemisia annua [1]. Historically it has been used in Chinese herbal medicine as an antipyretic and more recently as anti-malarial, but it also has been shown to have broad anti-neoplastic properties [2]. ART has been reported to induce apoptosis, differentiation and autophagy in colorectal cancer cells by impairing angiogenesis [3], inhibiting cell invasion and migration [4], inducing cell cycle arrest [5], upregulating ROS levels, regulating signal transduction [for example, activating the AMPK-mTOR-Unc-51-like autophagy activating kinase (ULK1) pathway in human bladder cancer cells [6] and blocking immune escape [7]. In addition, ART has been shown to restore the sensitivity of a number of cancer types to chemotherapeutic drugs by modulating various signaling pathways. For example, ART can improve the apoptosis of HCC by inhibiting the PI3K/AKT/mTOR pathway [8] and can increase liver cancer cell sensitivity to sorafenib via suppression of the MEK/ERK pathway [9]. For ovarian cancer specifically, ART has been shown to have clinical activity in treatment [10]. Although the amount of clinical data regarding the use of ART as an anticancer drug remains limited, preliminary results have been encouraging in terms of efficacy and tolerance [11-14].
Doxycycline is an FDA-approved drug, which first became available in 1967. It shows minimal side effects and is currently used world-wide as a broad-spectrum antibiotic. In recent years, pre-clinical and clinical data suggest that this tetracycline antibiotic could be repurposed to target, inhibit and eradicate cancer stem cells (CSCs) in multiple cancer types [15-16], particularly when used in combination with high-dose intravenous vitamin C (HDIVC) [15]. Preclinical data exists that doxycycline not only has an inhibitory effect on ovarian cancer, but also can increase sensitivity to cisplatin [17].
High dose intravenous vitamin C (HDIVC) has been evaluated as a potential treatment for cancer as an independent agent and in combination with standard chemotherapies. It was proposed to have anticancer effects as early as the 1950s, but earliest efforts to use high-dose vitamin C as a cancer treatment did not occur until the 1970s [18]. Two most studied mechanisms by which pharmacologic ascorbate concentrations have cytotoxic effects on tumor cells include increased pro-oxidant damage that is irreparable by tumor cells, and oxidation of ascorbate into dehydroascorbic acid (DHA), which is an unstable metabolite and can be cytotoxic [19]. In recent decades, data have been published that HDIVC up to 1.5g /kg/day appears to be well-tolerated [20], may improve the quality of life of terminal cancer patients [21], and reduce chemotherapy-associated toxicity in patients with ovarian cancer [23]. This led to a renewed interest in studying high-dose IV vitamin C as an anticancer treatment [20,22,24,25].
Comprehensive genomic profiling (CGP) is a next-generation sequencing (NGS) approach that uses a single assay to assess hundreds of genes including relevant cancer biomarkers, as established in guidelines and clinical trials, for therapy guidance [26]. While solid tumor DNA sequencing has been employed in conventional oncology for over two decades, liquid biopsies only gained traction in 2016, when FDA approved the first “liquid biopsy” test [27]. Evaluation of circulating tumor DNA (ctDNA) dynamics in advanced cancer patients is a real-time, precise, non-invasive method to assess treatment response and disease progression [28]. It is now possible to assess the efficacy of a new cancer treatment in as early as 4 weeks. This tool has revamped integrative oncology clinical research. Treatment response can now be assessed more rapidly and more frequently than the few and far between interval scans. Some tumor markers – CA19-9, CA27.29, CA125, PSA, CEA among others – have a long half-life and may not accurately reflect tumor growth or tumor burden [29].
Preliminary Data
Case 1
A 58-year-old, stage IC ovarian cancer at the time of diagnosis, presented 4.5 years later with small rise in CA 125. Patient was initially diagnosed in July 2017 with high-grade, serous ovarian cancer, pathologic stage pT1c pN0. At the time of diagnosis her CA 125 was 77.5 U/mL. After a robotic hysterectomy with bilateral salpingo-oophorectomy, she went on to receive 6 cycles of adjuvant carboplatin/paclitaxel, tolerated considerably well and completed November 2017.
For the next 4 years her CA 125 was never above 15.1 U/mL and regular surveillance imaging correlated with her NED status. In October 2019, roughly two years after completing adjuvant chemotherapy, she began high dose intravenous vitamin C (HDIVC), Trametes versicolor oral immunotherapy, prompted by consistently low natural killer cell function (< 20 LU10). For the next two years she was receiving these weekly at first, eventually reducing the frequency down to monthly. During this period, CA-125 levels were monitored at routine visits as illustrated in Fig. 1. At one of the routine visits on 9/29/21 it was noted that her CA-125 was now 25 U/mL, which subsequently elicited further investigation with ctDNA. Baseline Signatera was done on 9/29/21, the result was positive but very low at 0.03 MTM/mL (Figure 1).
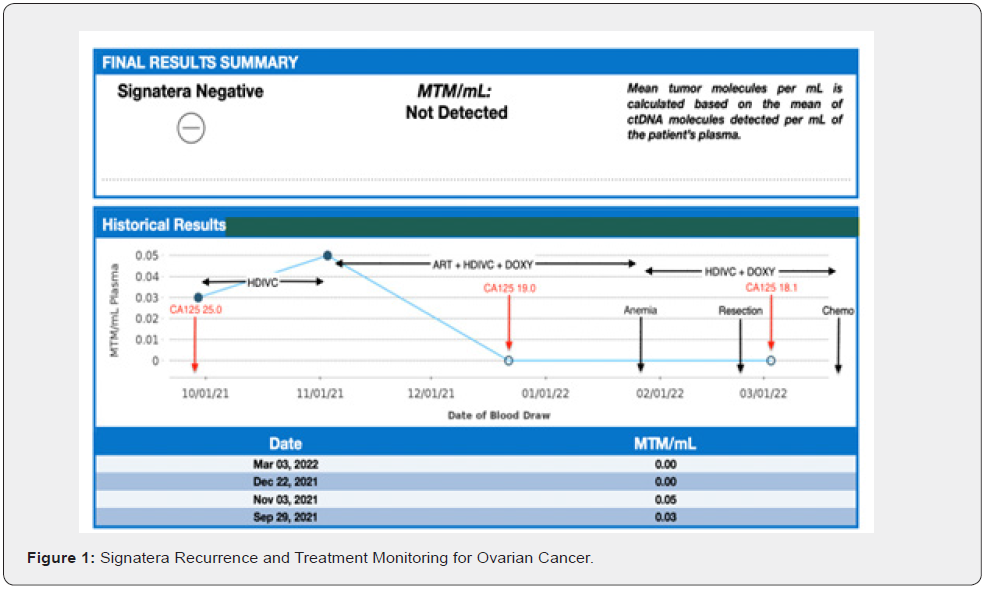
Biweekly HDIVC was re-initiated. Six-week-interval Signatera on 11/09/21 indicated a very slight increase to 0.05 MTM/mL (Figure 1). Subsequently, a therapeutic trial of parenteral HDIVC + Artesunate + Doxycycline Biweekly initiated for another 6 wk. Sixweek- interval Signatera testing on 01/04/2021 was now negative; 0.00 MTM/mL detected (Figure 1). Her CA125 on 12/22/21 had also decreased from 25 U/mL to 19 U/mL (Figure 2). CT/CAP and CT/PET scans performed in December 2021, revealed an enlarging hyper-metabolic right pelvic soft tissue nodule adjacent to the appendix, described as either peritoneal or a malignant pelvic lymph. On 2/23/22 she underwent laparoscopic resection of the right pelvic soft tissue mass, revealing an abnormal–appearing appendix, with firm nodular mass replacing the proximal third of the appendix, near the appendiceal/cecal junction. Pathology was consistent with high-grade serous carcinoma of gynecologic origin. Pelvic floor wash was negative. 3/3/22 CA-125 was 18.1 U/mL (Figure 1), Signatera, again, negative; 0.00 MTM/mL detected (Figure 1). Patient resumed HDIVC post-operatively and started adjuvant doxorubicin and carboplatin every 28 days, followed by PARPi. Of note, the patient did become anemic after 6 weeks of Artesunate. CBC from 1/26/22 significant for low RBC 3.47x10E6/uL, low Hgb 10.7 g/dL, low Hct 32.3%, all else within normal. Artesunate discontinued immediately and by 2/23/22 CBC was WNL.
Case 2
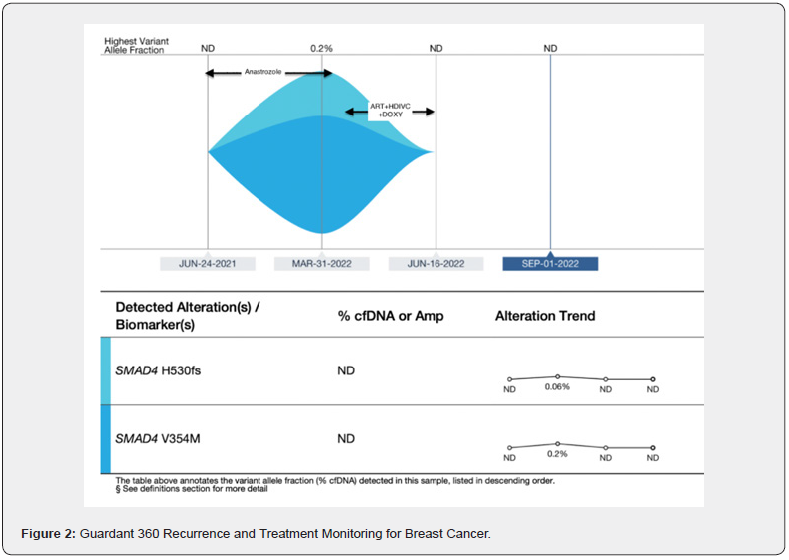
A 56-year-old postmenopausal cis-female with history of state IIA IDC of right breast estrogen receptor 95%, progesterone receptor 2%, HER-2 0%, Ki-67 9%, originally diagnosed September 2016. She is status post neoadjuvant chemotherapy with Dose Dense AC (DD-AC, Doxorubicin + Cyclophosphamide) and Dose Dense T (DD-T, Paclitaxel), bilateral mastectomy and right sentinel lymph node biopsy (1/3 SLN’s positive for macrometastasis). She declined adjuvant aromatase inhibitor (AI) plus ovarian function suppression (OFS) and was started on tamoxifen March 2017 but stopped after 6 months to 1 year. Unfortunately, right breast MRI in 4/2021 revealed a suspicious right breast mass along with bilateral axillary lymphadenopathy. June 2021 liquid biopsy via Guardant 360 released no reportable tumor-related somatic alterations (Figure 2). US-guided right axillary biopsy August 2021 was consistent with breast primary metastatic carcinoma. She declined additional axillary surgery and radiation. While considering her treatment options, she started anastrozole April 2021 and continued it through April 2022. March 2022 liquid biopsy via Guardant 360 detected 0.26% ctDNA with mutations for SMAD4 tumor-related somatic alterations (Figure 2). She was started on triple IV therapy (Artesunate, ascorbate, and doxycycline) and completed 14 IV infusions twice per week over months of May 2022 to June 2022. Interval Guardant 360 on 6/16/22 had no reportable tumor-related somatic alterations (Figure 2). Guardant 360 was repeated again on 9/1/22, again, showing no reportable tumor-related somatic alterations (Figure 2).
Conclusion
Four-6 weeks of triple IV therapy with Artenusate + ascorbate + doxycycline can eradicate evidence of circulating tumor cells and circulating tumor DNA. We propose that this triple therapy may be useful in early recurrence of disease in early stage ovarian and breast cancer.
References
- Golenser J, Waknine JH, Krugliak M, Hunt NH, Grau GE (2006) Current perspectives on the mechanism of action of artemisinins. Int J Parasitol 36(14): 1427–1441.
- Crespo-Ortiz MP, Wei MQ (2012) Antitumor activity of artemisinin and its deriva-tives: From a well-known antimalarial agent to a potential anticancer drug. J Biomed Biotechnol 2012: 247597.
- Verma S, Das P, Kumar VL (2017) Chemoprevention by artesunate in a preclinical model of colorectal cancer involves down regulation of β-catenin, suppression of angiogenesis, cellular proliferation and induction of apoptosis. Chem Biol Interact 278: 84-91.
- Ma JD, Jing J, Wang JW, Yan T, Li QH, et al. (2019) A novel function of artesunate on inhibiting migration and invasion of fibroblast-like synoviocytes from rheumatoid arthritis patients. Arthritis Res Ther 21(1): 1532019.
- Markowitsch SD, Schupp P, Lauckner J, Vakhrusheva O, Slade KS, et al. (2020) Artesunate inhibits growth of sunitinib-resistant renal cell carcinoma cells through cell cycle arrest and induction of ferroptosis. Cancers (Basel) 12(11): 31502020.
- Zhou X, Chen Y, Wang F, Wu H, Zhang Y, et al. (2020) Artesunate induces autophagy dependent apoptosis through upregulating ROS and activating AMPK-mTOR-ULK1 axis in human bladder cancer cells. Chem Biol Interact 331: 1092732020.
- Qian P, Zhang YW, Zhou ZH, Liu JQ, Yue SY, et al. (2018) Artesunate enhances γδ T-cell-mediated antitumor activity through augmenting γδ T-cell function and reversing immune escape of HepG2 cells. Immunopharmacol Immunotoxicol 40(2): 107–116.
- Jing W, Shuo L, Yingru X, Min M, Runpeng Z, et al. (2019) Artesunate promotes sensitivity to sorafenib in hepatocellular carcinoma. Biochem Biophys Res Commun 519(1): 41–45.
- He W, Huang X, Berges BK, Wang Y, An N, et al. (2021) Artesunate regulates neurite outgrowth inhibitor protein B receptor to overcome resistance to sorafenib in hepatocellular carcinoma cells. Front Pharmacol 12: 6158892021.
- Greenshields AL, Shepherd TG, Hoskin DW (2017) Contribution of reactive oxygen species to ovarian cancer cell growth arrest and killing by the anti-malarial drug artesunate. Mol Carcinog 56(1): 75–93.
- McDowell A Jr, Hill KS, McCorkle JR, Gorski J, Zhang Y, et al. (2021) Preclinical Evaluation of Artesunate as an Antineoplastic Agent in Ovarian Cancer Treatment. Diagnostics 11(3): 395.
- Yang X, Zheng Y, Liu L, Huang J, Wang F, et al. (2021) Progress on the study of the anticancer effects of artesunate (Review). Oncology Letters 22(5): 750.
- Cangcang Xu, Huihui Zhang, Lingli Mu, Xiaoping Yang (2020) Artemisinins as Anticancer Drugs: Novel Therapeutic Approaches, Molecular Mechanisms, and Clinical Trials. Front Pharmacol 11: 529881.
- Efferth T, Dunstan H, Sauerbrey A, Miyachi H, Chitambar CR (2001) The anti-malarial artesunate is also active against cancer. Int J Oncol 18(4): 767-773.
- Ernestina Marianna De Francesco, Gloria Bonuccelli , Marcello Maggiolini , Federica Sotgia , Michael P Lisanti, et al. (2017) Vitamin C and Doxycycline: A synthetic lethal combination therapy targeting metabolic flexibility in cancer stem cells (CSCs). Oncotarget 8(40): 67269-67286.
- Cristian Scatena, Manuela Roncella, Antonello Di Paolo, Paolo Aretini , Michele Menicagli, et al. (2018) Doxycycline, an Inhibitor of Mitochondrial Biogenesis, Effectively Reduces Cancer Stem Cells (CSCs) in Early Breast Cancer Patients: A Clinical Pilot Study. Front Oncol 8: 452.
- Wu W, Yu Lh, Ma B, Xu Mj (2014) The Inhibitory Effect of Doxycycline on Cisplatin-Sensitive and -Resistant Epithelial Ovarian Cancer. PLOS ONE 9(3): e89841.
- Nauman G, Gray JC, Parkinson R, Levine M, Paller CJ (2018) Systematic Review of Intravenous Ascorbate in Cancer Clinical Trials. Antioxidants 7(7): E89.
- Ohno S, Ohno Y, Suzuki N, Soma G, Inoue M (2009) High-dose vitamin C (ascorbic acid) therapy in the treatment of patients with advanced cancer. Anticancer Res 29(3): 809–815.
- Hoffer LJ, Levine M, Assouline S, D Melnychuk, S J Padayatty, et al. (2008) Phase I clinical trial of i.v. ascorbic acid in advanced malignancy. Ann Oncol 19(11): 1969-1974.
- Yeom CH, Jung GC, Song KJ (2007) Changes of terminal cancer patients’ health-related quality of life after high dose vitamin C administration. J Korean Med Sci 22(1): 7-11.
- Padayatty SJ, Riordan HD, Hewitt SM, Arie Katz, L John Hoffer, et al. (2006) Intravenously administered vitamin C as cancer therapy: three cases. CMAJ : Canadian Medical Association journal, journal de l’Association medicale Canadienne 174(7): 937-942.
- Ma Y, Chapman J, Levine M (2014) High-Dose Parenteral Ascorbate Enhanced Chemosensitivity of Ovarian Cancer and Reduced Toxicity of Chemotherapy. Sci Transl Med 6(222): 222ra18.
- Klimant E, Wright H, Rubin D, Seely D, Markman M (2018) Intravenous vitamin C in the supportive care of cancer patients: a review and rational approach. Curr Oncol 25(2): 139-148.
- Gonzalez MJ, Miranda-Massari (2014) New Insights on Vitamin C and Cancer. Springer Briefs in Cancer Research. Springer.
- Michael F Berger, Elaine R Mardis (2018) The emerging clinical relevance of genomics in cancer medicine. Nature reviews. Nat Rev Clin Oncol 15(6): 353-365.
- Kwapisz D (2017) The first liquid biopsy test approved. Is it a new era of mutation testing for non-small cell lung cancer? Annals of translational medicine 5(3): 46.
- Qu Zhang JL, Song Wu, Han Si, Chen Gao, Wenjing Xu, et al. (2020) Prognostic and predictive impact of circulating tumor DNA in patients with advanced cancers treated with immune checkpoint blockade. Cancer Discovery 10(12): 1842-1853.
- Sevil E Inanc, R M, Emin Darendeliler, Vildan Yasasever, Haluk Onat (1999) Significance of Marker Half-life during Chemotherapy in Non-seminomatous Germ Cell Testicular Tumors. Acta Oncologica 38(4): 505-509.






























