The Metamorphosed Wattle-Lobular Carcinoma in Situ
Anubha Bajaj*
Histopathologist in A B Diagnostics, New Delhi, India
Submission: September 22, 2022; Published: October 03, 2022
*Corresponding Address: Anubha Bajaj, Histopathologist in A B Diagnostics, New Delhi, India
How to cite this article: Anubha B. The Metamorphosed Wattle-Lobular Carcinoma in Situ. Canc Therapy & Oncol Int J. 2022; 22(3): 556089. DOI:10.19080/CTOIJ.2022.22.556089
Mini Review
Lobular carcinoma in situ (LCIS) is a non-invasive, neoplastic proliferation of uniform, miniature, dys-cohesive cells originating from terminal duct lobular unit (TDLU). Consequently, permeation and distension of majority of breast acini configuring the incriminated mammary lobule may ensue. Lobular neoplasia (LN) of breast is comprised of atypical lobular neoplasia (ALH) and lobular carcinoma in situ (LCIS). Classic LCIS exhibits permeation and expansion of >50% of mammary acini with neoplastic cells whereas ALH enunciates < 50% of TDLU pervaded with tumour cells [1,2]. Classic LCIS (c-LCIS) is constituted of type A or type B epithelial cells demonstrating varied morphology. Non classical LCIS is comprised of pleomorphic subtype and florid subtype [1,2].
Classic LCIS represents with lobulo-centric proliferation of monomorphic tumour cells which distend mammary lobular units. Pagetoid involvement of terminal ducts may or may not ensue [1,2]. Characteristically, loosely cohesive, uniformly distributed neoplastic cells, designated as ‘marbles in a bag’ are discerned. Tumour cells are mildly enlarged with indistinct cellular perimeter, pale cytoplasm, regular, miniature nuclei, well distributed nuclear chromatin and inconspicuous nucleoli [1,2]. Classic LCIS manifests as a contributor to and non-obligate precursor of invasive carcinoma breast. Categorization of tumour in situ (Tis) is no longer applicable to classic LCIS [1,2]. Neoplasm is induced by dysfunctional E-cadherin catenin axis which engenders loss of cellular cohesion. Decimated E-cadherin protein occurs due to genetic mutation, deletion, or methylation. Aberrant expression of E-cadherin may concur [1,2].
Although classic LCIS is commonly discerned in premenopausal women, tumour emergence may occur up to 83 years with a mean age of neoplastic detection at ~ 53.7 years [1,2]. Generally, classic LCIS demonstrates multiple foci within ipsilateral or bilateral breasts. Multi-centric lesions confined to a singular breast are common although bilateral breasts are frequently incriminated.
Classic LCIS can exceptionally implicate ectopic breast tissue [1,2]. Classic LCIS enunciates genetic mutation of E-cadherin with loss of wild type allele due to loss of heterozygosity (LOH) which manifest as preliminary genomic modifications engendering the neoplasm [1,2].
E-cadherin mediates intracellular adhesion with cellular polarity and contributes to maintenance of lobular breast architecture. The molecule may prohibit growth and infiltration of neoplastic cells [1,2]. Deficient E-cadherin induces decimated cell to cell cohesion, amplifies cellular proliferation and alters organization of breast lobules with characteristic emergence of lobular neoplasia [1,2]. Factors contributing to emergence of classic LCIS simulate factors influencing DCIS or invasive carcinoma breast and are denominated as ~hormonal exposure as majority of LCIS exemplify an intense, diffuse expression of oestrogen receptors. ~enhanced possible occurrence of LCIS is associated with family history of carcinoma breast, previously benign mammary lesions detected within surgical samples, nulliparous subjects, elderly primigravida, delayed menopause, intensely dense breasts upon mammography and postmenopausal women treated with excessive, unopposed oestrogen replacement therapy [1,2]. LCIS is denominated as a preliminary, clonal, low grade, neoplastic epithelial proliferation demonstrating several copy number genomic aberrations and somatic genetic mutations concomitant with invasive lobular carcinoma (ILC) [1,2].
In fact, LCIS associated with ILC delineates significant genetic alterations, in contrast to pure LCIS [1,2]. Frequently, copy number alterations such as loss of 16q and gain of 1q chromosomes are observed. Besides, loss of 16q and gain of 1q may concur with various mammary epithelial proliferations immune reactive to oestrogen receptors such as columnar cell lesions or well differentiated invasive ductal carcinoma with variants as tubular carcinoma or low-grade DCIS [1,2]. Thus, LCIS is contemplated to be induced by a low grade, oestrogen receptor imbued pathway of breast carcinogenesis. Lobular lesions demonstrate CDH1 tumour suppressor gene encoding for cell adhesion molecule E-cadherin which is mapped at chromosome 16q22.1. Loss of 16q is frequently accompanied by inactivating mutations, homozygous deletions or gene promoter methylation of CDH1 with consequent bi-allelic genetic silencing [1,2]. Diverse genetic alterations and combinations may engender a varied immunoreactivity. Also, LCIS is devoid of various tumour suppressor genes situated upon chromosome 16 [1,2]. CCCTC binding factor (CTCF) gene and dipeptidase 1 (DPEP1) gene may be incriminated in tumorigenesis. CDH1 is a frequent somatic genetic mutation followed by PIK3CA and CBFB. Genomic mutations of p53 and PTEN are exceptional [1,2].
Penetrant germline mutations of CDH1 exhibit enhanced possible emergence of hereditary diffuse gastric carcinoma and lobular carcinoma, especially bilateral LCIS. In contrast to classic LCIS, pleomorphic LCIS and florid LCIS delineate elevated genomic instability with increased copy number alterations and genetic amplification [1,2]. LCIS is devoid of specific clinical symptoms or imaging features. Neoplasm is identified upon surgical tissue sampling or surgical excision of breast for unrelated factors [1,2]. Exceptionally, < 2% of classic LCIS can be subjected to surgical tissue sampling due to associated anomalies detected upon imaging such as aggregated, amorphous or granular calcification upon mammography, shadowed, avascular, irregular, hypoechoic mass upon ultrasonography or heterogeneous, non-tumorous image enhancement with persistent enhancement kinetics upon magnetic resonance imaging (MRI). LCIS may infrequently be discerned following surgical tissue sampling for palpable breast mass or nipple discharge [1,2].
Upon cytological assessment, aspirate from classic LCIS is cellular and composed of loosely cohesive clusters and aggregates of miniature, uniform, and bland neoplastic cells. Tumour cells display intracytoplasmic lumen with eccentric, mildly enlarged nuclei and miniature, prominent nucleoli. Non-atypical, spindleshaped myoepithelial cells may appear along with classic LCIS cells [1,2]. Cellular aspirate composed of signet ring cells admixed with dis-cohesive fragments of lobular epithelium may indicate classic LCIS. However, cytological distinction between classic LCIS and invasive lobular carcinoma (ILC) may be challenging. Significant (> 50%) aspirates of classic LCIS may be contemplated as benign or non-representative [1,2].
Upon gross examination, classic LCIS appears non palpable and gross abnormalities of incriminated breast are absent. Gross anomalies may ensue due to coexistent mammary lesions or proliferative alterations as encountered with configured cysts or nodular, firm to hard breast parenchyma [1,2]. Upon microscopy, LCIS incriminates terminal duct lobular unit (TDLU) with occupancy and distension of breast acini. LCIS exhibits occupation and expansion of around > 50% of acini configuring a TDLU. Lesions implicating <50% of acini within a TDLU are designated as atypical lobular hyperplasia [1,2]. Lobular distension is denominated as representation of ≥ 8 cells confined to cross sectional diameter of an acinus. Atypical lobular hyperplasia (ALH) partially incriminates mammary lobule with occupation of acini in the absence of significant lobular distention. LCIS predominantly involves mammary lobules although basement membrane of ducts may exhibit ‘pagetoid’ dissemination of tumour cells. Pagetoid dissemination within ducts is characteristically discerned between luminal and myoepithelial layers of duct epithelium in the absence of disintegration of ductal epithelium or occupancy of ductal lumen. Incriminated duct frequently appears convoluted, designated as ‘cloverleaf’ pattern [1,2]. LCIS may emerge as secondary to lesions such as sclerosing adenosis, radial scar, fibroadenoma, collagenous spherulosis or papilloma.
‘Rosen triad’ is configured by concurrence of tubular carcinoma, columnar cell lesions and LCIS or atypical lobular hyperplasia (ALH). Classic LCIS is comprised of monomorphic, uniformly spaced, loosely cohesive cells devoid of polarization or glandular configuration. Classic LCIS appears to lack significant nuclear pleomorphism or mitotic activity. Exceptionally, classic LCIS may depict apoptosis of singular cells or minute foci of necrosis although comedo necrosis is characteristically absent [1,2]. Comedo necrosis is infrequently discerned in classic LCIS in accompaniment with characteristic cytological and architectural features. Lesions borderline between classic LCIS configured of type B cells and pleomorphic LCIS and may be categorized as classic LCIS composed of type B cells [1,2]. Lesions with extensive LCIS, cytological features of classic LCIS and borderline distension of acini between classic LCIS and florid LCIS can be designated as classic LCIS [1,2].
LCIS is constituted of dual subtypes of cells as •type A cells depicting miniature to minimally enlarged, uniform, spherical nuclei or inconspicuous nucleoli. •type B cells incorporated with abundant cytoplasm, enlarged nuclei and prominent nucleoli. Type A and type B cells may coexist within a singular lesion [1,2]. LCIS cells typically delineate pale to slightly eosinophilic cytoplasm and indistinct cellular perimeter [1,2]. Few cells configuring LCIS may demonstrate intracytoplasmic vacuoles or lumina and appear imbued with an eosinophilic globule. Subtle intracytoplasmic vacuoles require special histochemical stains for highlighting mucin [1,2]. Alternatively, enlarged mucin vacuoles may propel the nucleus against cellular membrane to configure signet ring cells [1,2]. Extrinsic myoepithelial cell layer appears attenuated although retained within implicated acini and ducts. Scattered myoepithelial cells may be admixed with neoplastic epithelial cells confined to incriminated ducts and acini [1,2]. Myoepithelial cells are minimally immune reactive to E-cadherin on account of cross-reactive P-cadherin [1,2].
LCIS is constituted of dual subtypes of cells as •type A cells Cyto-architectural features of LCIS appear to diverge from classic LCIS. Tumour cells enunciate distinct cellular membrane with occurrence of apocrine cells or clear cell alterations or exhibit myoid cells with intensely stained, eosinophilic cytoplasm and eccentric nuclei. Pleomorphic LCIS is constituted of enlarged cells incorporated with enlarged, pleomorphic nuclei. Centric tumour necrosis and apocrine metaplasia may be discerned [1,2]. Florid LCIS along with or devoid of centric necrosis, is configured of cytological features of classic LCIS accompanied by significantly distended ducts disseminated within solid tumour architecture [1,2] (Figures 1 & 2).
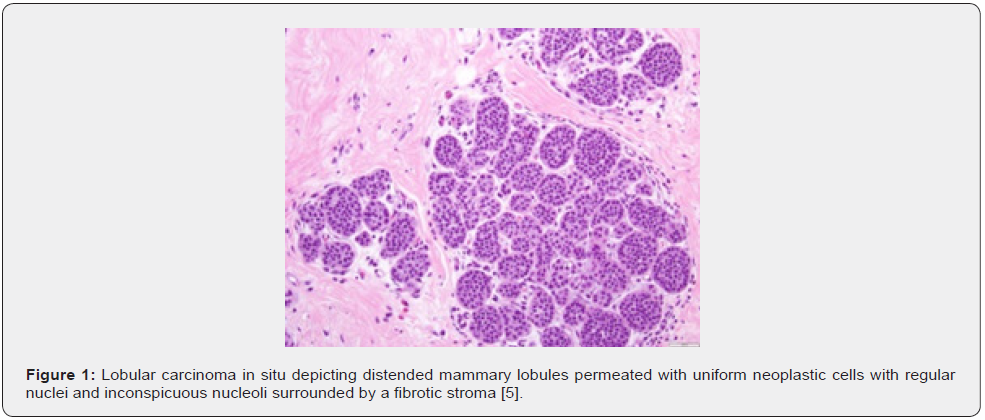
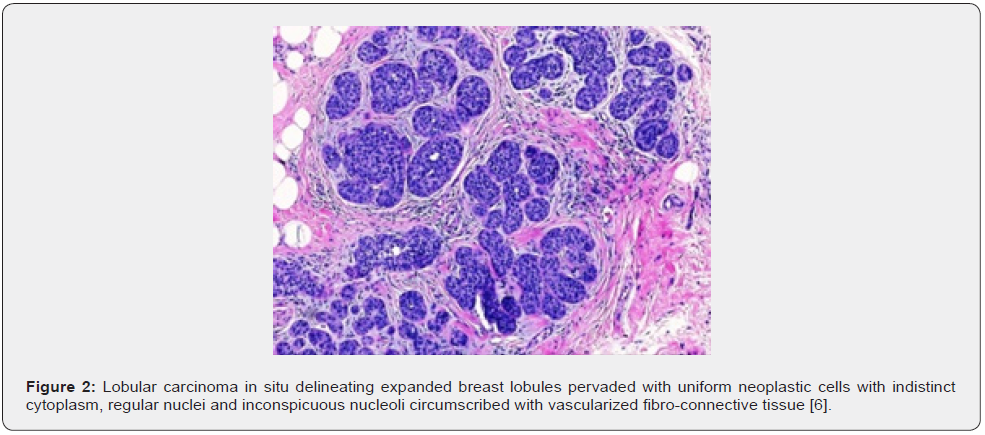
TNM staging of lobular breast carcinoma is denominated as •stage 0 where neoplastic cells are confined within breast lobule devoid of invasion of stroma or circumscribing adipose tissue, designated as lobular carcinoma in situ, a non-invasive carcinoma breast. Metastasis to regional lymph nodes or distant organs is absent [2,3]. •stage I A where tumefaction is ≤ 2centimetres and devoid of regional lymph node or distant metastasis. ~stage IB where tumour is ≤2-centimetre magnitude along with micrometastasis within one to three axillary lymph nodes, designated as metastasis between 0.2 millimetre to 2.0-millimetre diameter or >200 cells. Distant metastasis is absent [2,3]. •stage II A where distant metastasis is absent and ~tumour is non discernible or ≤2-centimetre diameter with metastasis into one to three axillary nodes of >2millimetre magnitude [2,3]. ~tumour is ≤2 centimetre diameter with miniature deposits into internal mammary lymph nodes detected upon sentinel lymph node dissection. ~tumour is non discernible or ≤ 2-centimetre diameter with metastasis into one to three axillary nodes and internal mammary lymph nodes detected upon sentinel lymph node biopsy. ~tumour is between 2 centimetre to 5-centimetre magnitude with absent regional lymph node metastasis [2,3].
•stage IIB where distant metastasis is absent and ~tumour is between 2 centimetres and 5 centimetres with metastasis into one to three axillary nodes and internal mammary lymph nodes detected upon sentinel lymph node biopsy. ~tumour is > 5 centimetres with absent regional lymph node metastasis. Metastasis into chest wall or thoracic cutaneous surface is absent [2,3]. •stage IIIA where distant metastasis is absent and ~tumour is non discernible or ≤5-centimetre magnitude with metastasis into four to nine axillary lymph nodes or enlarged internal mammary lymph nodes. ~tumour is ≥5-centimetre magnitude with metastasis into one to nine axillary lymph nodes or internal mammary nodes. Metastasis into chest wall or thoracic cutaneous surface is absent [2,3].
•Stage IIIB where tumour extends into chest wall or cutaneous surface with absence of distant metastasis along with ~metastasis into regional lymph nodes is absent. ~metastasis into one to three axillary lymph nodes or miniature deposits into internal mammary lymph nodes detected upon sentinel lymph node biopsy. ~metastasis into four to nine axillary lymph nodes or enlarged internal mammary lymph nodes [2,3]. •stage IIIC where tumour is non discernible or exhibits variable magnitude with absent distant metastasis along with ~metastasis into ≥10 axillary lymph nodes. ~metastasis into infra-clavicular lymph nodes.
~Metastasis into supra-clavicular lymph nodes. ~metastasis into axillary lymph nodes with enlarged internal mammary lymph nodes [2,3]. ~metastasis into ≥4 axillary lymph nodes and miniature deposits within internal mammary lymph nodes detected upon sentinel lymph node biopsy. •stage IV where tumour magnitude is variable and regional lymph node metastasis may or may not appear. Distant metastasis into diverse viscera as bone, brain, hepatic or pulmonary parenchyma or distant lymph nodes are discerned [2,3].
Lobular carcinoma in situ is immune reactive to oestrogen receptors, progesterone receptors or p120 catenin. Ki67 proliferative index is minimal. Intracytoplasmic mucin can be emphasized with special stains as mucin, alcian blue or mucicarmine [3,4]. Lobular carcinoma in situ is immune nonreactive to E-cadherin, β-catenin or HER2/neu [3,4].
Lobular carcinoma in situ necessitates segregation from neoplasms such as atypical lobular hyperplasia demonstrating artefactual dis-cohesion, intraepithelial histiocytes, myoepithelial hyperplasia or clear cell change, variants of apocrine metaplasia, pseudo-lactational hyperplasia, invasive ductal carcinoma with lobular features, pre-existing sclerosing lesion as sclerosing adenosis, radial scar, complex sclerosing lesion, solid or alveolar pattern of invasive lobular carcinoma, pleomorphic lobular carcinoma in situ, ductal carcinoma in situ (DCIS) with variants as solid, low grade DCIS, cancerization of lobules with DCIS, cribriform DCIS or collagenous spherulosis with accompanying LCIS, pagetoid LCIS versus pagetoid DCIS, LCIS with comedo necrosis versus DCIS with comedo necrosis, in situ carcinoma with mixed ductal and lobular carcinoma, gastric signet ring carcinoma metastatic to breast, sclerosing epithelioid fibrosarcoma, leukemic and lymphomatous incrimination of breast, Rosai-Dorfman disease or granular cell tumour.
Classic LCIS is discovered as an incidental neoplasm within surgical tissue samples performed for various indications as screen detected calcification or mass-producing lesions [3,4]. Columnar cell lesions indicate or are associated with LCIS and frequently depict calcification discerned upon mammography. LCIS or DCIS can be appropriately distinguished upon morphological assessment or with E-cadherin and p120 catenin immunostaining.
Classic LCIS is devoid of characteristic radiologic features [3,4]. Mammography may be unremarkable. Few instances demonstrate a distinct tumefaction or calcification [3,4]. Upon ultrasonography, an avascular, irregular, poorly defined, hypoechoic tumefaction with posterior shadowing may be discerned. Magnetic resonance imaging exhibits heterogeneous, non-tumorous image enhancement with delayed persistent enhancement kinetics. Micro-calcifications are uncommon. However, miniature, focal calcifications may be associated with LCIS [3,4]. Calcification is common in LCIS coexistent with sclerosing adenosis, columnar cell changes or hyperplasia, atrophic mammary lobules or ducts and collagenous spherulosis [3,4]. ‘ Mirror biopsy’ of contralateral breast is not a recommended or adopted procedure.
Classic LCIS discovered incidentally upon percutaneous image guided core needle biopsy may not be benefitted by surgical extermination. Surgical tissue sampling is recommended where core needle biopsy detects classic LCIS in association with various proliferative lesions of breast as radial scar or atypical ductal hyperplasia or instances where histological and imaging features appear discordant [3,4]. Classic LCIS can be optimally managed with active monitoring and chemoprevention with antioestrogens. Non-classical LCIS detected upon core needle tissue sampling mandates surgical eradication of neoplasm. Obtaining a tumour free surgical perimeter within excised specimens of classic LCIS remains unnecessary and not recommended [3,4].
Carriers of BRCA1 or BRCA2 genetic mutation can be subjected to prophylactic mastectomy which appears efficacious in enhancing overall survival [3,4]. LCIS is associated with ~10 times enhanced possible emergence of carcinoma breast. In contrast to contralateral breast, ipsilateral breast frequently depicts emergence of invasive carcinoma breast, probably within ~ 30 years [3,4]. Although frequently comprised of invasive carcinoma breast –not otherwise specified (NOS), invasive mammary carcinoma detected after classic LCIS may be constituted of lobular carcinoma. Clinical and histological factors contributing to metamorphosis of LCIS into invasive carcinoma breast remain inconsistent and obscure [3,4]. Occurrence of LCIS upon margins of surgical resection appears non-contributory to enhanced localized tumour reoccurrence [3,4]. Ductal carcinoma in situ with concurrent lobular neoplasia subjected to breast conservation therapy exhibits augmented possible reoccurrence of carcinoma breast within ipsilateral breast [3,4].
References
- Limaiem F, Khan M (2022) Lobular Breast Carcinoma. Stat Pearls International 2022. Treasure Island, Florida, USA.
- Braasch MC, Amin AL, Christa R Balanoff, Jamie L Wagner, Kelsey E Larson, et al. (2022) Prognostic Significance of Lobular Carcinoma In-Situ (LCIS) Diagnosed Alongside Invasive Breast Cancer. Breast Cancer (Auckl) 16: 11782234211070217.
- Brogi E (2022) The morphologic spectrum of lobular carcinoma in situ (LCIS) observations on clinical significance, management implications and diagnostic pitfalls of classic, florid and pleomorphic LCIS. Virchows Arch.
- Mallory MA, Whiting K, Anna Park, Mithat Gonen, et al. (2022) Synchronous and metachronous bilateral breast cancer among women with a history of lobular carcinoma in situ. Breast Cancer Res Treat 194(1): 137-148.
- Image 1 Courtesy: Surgical pathology clinics
- Image 2 Courtesy: Wikimedia commons






























