Comparison of Trolamine Timing in Breast Cancer Radiotherapy to Prevent from Radiation Dermatitis: A Single Center Study
Bora Uysal*, Hakan Gamsiz, Cemal Ugur Dursun and Murat Beyzadeoglu
University of Health Sciences, Gulhane Medical Faculty, Department of Radiation Oncology, Ankara, Turkey
Submission: August 29, 2022; Published: September 09, 2022
*Corresponding Address: Bora Uysal, , MD, Asc. Professor of Radiation Oncology, University of Health Sciences, Gulhane Medical Faculty, Department of Radiation Oncology, 06010, Ankara, Turkey
How to cite this article: Bora U, Hakan G, Cemal U D, Murat B. Comparison of Trolamine Timing in Breast Cancer Radiotherapy to Prevent from Radiation Dermatitis: A Single Center Study. Canc Therapy & Oncol Int J. 2022; 22(3): 556086. DOI:10.19080/CTOIJ.2022.22.556086
Abstract
Objectıve: We compared the initial usage of trolamine at the start of radiotherapy with second week of radiotherapy in breast cancer patients.
AIMS: Primary outcomes were the toxicity scores between two groups and secondary outcomes were the quality of life in patients.
Subjects and Methods: 41 patients with breast cancer receiving adjuvant RT between November 2020 and October 2021 at our department were included in this study. Eligibility criteria included ≤80 year of age, Eastern Cooperative Oncology Group performance status of 0–1, and no previous history of breast RT. Two groups were compared with regard to trolamine usage starting at the first day of treatment or after 2 weeks of radiotherapy. The first group consisted of 20 patients and the second group included 21 patients.
Results: 20 of 41 patients had grade 0 toxicity whereas 15 of all experienced grade 1 and remaining 6 of them had grade 2 toxicity. 16 patients of 20 who started trolamine at the first day of treatment had grade 0 toxicity whereas 3 of 20 had grade 1 and 1 of all had grade 2 toxicity. 5 patients of 21 who started trolamine after 2 weeks of radiotherapy had grade 0 toxicity whereas 12 of 21 had grade 1 and 5 of all had grade 2 toxicity. No patient in either group experienced grade 3 radiodermatitis. Association of comorbid diseases, smoking or alcohol intake and toxicity was statistically nonsignificant (p=0.13).
Conclusion: Trolamine usage from the inital day of radiotherapy was resulted in clinically and statistically significantly lesser toxicity compared to the group who started trolamine after 2 weeks of radiotherapy (p=0.011).
Introduction
Radiation dermatitis is the most common effect of especially breast cancer and head and neck cancer patients treated with radiotherapy. Radiosensitive skin cells is damaged and loss their partial basal layer via fractionation and repeated doses of radiotherapy. Mild erythema, dry or moist desquamation, ulcerations may be start after two weeks of radiotherapy. Pain, itching, acneiform lesions, skin peeling are the symptoms of local discomfort in patients treated with radiotherapy. More than 50% of patients whom were delivered radiotherapy encounter with moderate to severe skin reactions. Total radiation dose, fractionation, tissue or organ treated, comorbid diseases, concomitant chemotherapy or immunotherapy are the different factors causing radiation dermatitis. Radiotherapy damages DNA with unilateral or double strand breaks causing tissue destruction. Epidermal and dermal inflammatory responses start the period of radiation dermatitis which rarely results with scin necrosis.
Quality of life is affected in some of the patients whom treated with radiotherapy. No guideline or management algorithm exist for radiation dermatitis but also recommendations or institute protocols are used by treating physician. Alternative skin products are used to manage dermal reactions due to radiotherapy. Prevention and also treatment of skin reactions are the main goal of topical agents. Trolamine has been used especially in USA and Europe Region more than 30 years. Macrophages are activated and promotion of granulation tissue is reorganized after trolamine application. Trolamine is similar with nonsteroidal antiinflammatory products in topical management. It is considered as safe and tolerable with its effects of reducing pain, itching and eryhtema. Steroidal agents, nonsteroidal compounds and hydrating topicals are still used for radiation dermatitis [1].
Material and Methods
41 patients with breast cancer receiving adjuvant RT between November 2020 and October 2021 at our department were included in this study. Eligibility criteria included ≤80 year of age, Eastern Cooperative Oncology Group performance status of 0–1, and no previous history of breast RT. Informed consents of all patients were obtained. Computed tomography (CT)-simulation images were acquired for each patient as per our institutional protocol. Breast target volumes either breast or sternal wall and relevant critical structures were delineated on CT-simulation images. RT planning was performed by using the PrecisePLAN (Elekta, UK) Treatment Planning System. Prescribed whole breast dose was 50 Gy in 25 fractions either followed by a tumor bed boost of additional 10 Gy in 5 fractions or no boost, 2.66 Gy per fractions in 15 total day with 10 Gy boost as a hypofractionation according to the stage, patient’s choice or age etc.
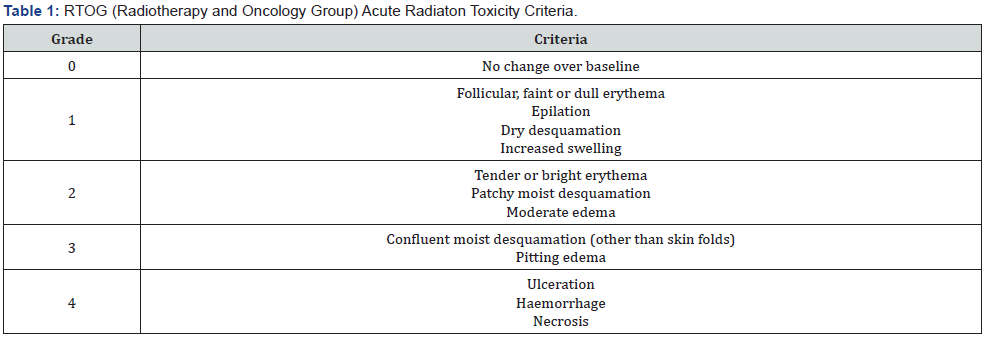
The linear accelerator (Synergy, Elekta, UK) available at our department was used for treatment delivery. Two groups were compared with regard to trolamine usage starting at the first day of treatment or after 2 weeks of radiotherapy. The first group consisted of 20 patients and the second group included 21 patients. Age, sex, primary pathological subtype, grade, receptor status, radiotherapy portal, dose, toxicity score, comorbid disease, status of smoking and alcohol intake were recorded. RTOG (radiation therapy and oncology group) toxicity score scale was used for analysis between these two groups (Table 1). Patients were followed up weekly after the start till the end of the treatment.
Statıstıcal Analysis
In descriptive statistics, mean and standard deviation were used for normally distributed variables, which were analyzed using the paired t-test, and median (minimum–maximum) was used for nonnormally distributed variables, which were analyzed using the Wilcoxon signed rank test. Statistical Package for the Social Sciences, version 15.0 (SPSS, Inc. Chicago, IL) software was used for analysis and the level of significance was set at p < 0.05.
Results
All 41 (100%) patients were female. Pathological subtypes were 1 DCIS (ductal carcinoma in situ), 6 mixt type, 2 mucinous and 32 ductal adenocarcinoma respectively. Mean age was 54 (28-79) years. 5 patients were grade 1, 26 of all were grade 2 and remaining 10 patients were grade 3 breast cancer. 4 patients were triple negative and 1 patient was triple positive. 32 patients were luminal A and 4 patients were luminal B. 10 patients were surgically treated with modified radical mastectomy and remaining 31 of all were treated with breast conserving surgery. 15 patients were delivered 2.66 Gy in 15 fractions and 10 Gy boost whereas 15 patients were delivered 50 Gy and 10 Gy boost in 2 Gy per fractions and remaining 11 patients were received 50 Gy.
20 of 41 patients had grade 0 toxicity whereas 15 of all experienced grade 1 and remaining 6 of them had grade 2 toxicity. 16 patients of 20 who started trolamine at the first day of treatment had grade 0 toxicity whereas 3 of 20 had grade 1 and 1 of all had grade 2 toxicity. 5 patients of 21 who started trolamine after 2 weeks of radiotherapy had grade 0 toxicity whereas 12 of 21 had grade 1 and 5 of all had grade 2 toxicity. No patient in either group experienced grade 3 radiodermatitis. Association of comorbid diseases, smoking or alcohol intake and toxicity was statistically nonsignificant (p=0.13). Trolamine usage from the inital day of radiotherapy was resulted in clinically and statistically significantly lesser toxicity compared to the group who started trolamine after 2 weeks of radiotherapy (p=0.011).
Discussion
Aquaphor, topical cream, gel, corticosteroid, trolamine and any other different agents has been used for prevention and treatment of radiation dermatitis induced by radiotherapy. Bensadoun et al demonstrated that trolamine might reduce the ratio of dermatitis after chemoradiotherapy of head and neck squamous cancer patients [2]. Wang et al. [3] showed similar results about radiation or chemotherapy induced dermatitis. Human skin cell lines were applied with trolamine in a study by Boisnic et al. Vasodilatation, dermal edema were diminished by trolamine in this study.
Thermal burns are the first application region for therapy indication suggesting that accelerated repair ratio of damaged skin. Trolamine is considered beneficial for grade 3 or 4 radiation dermatitis because of its positive effect over healing process [4]. Elliot et al showed no advantage of trolamine about radiation dermatitis incidence or quality of life in the patients of RTOG 9913 trial [5]. However this results were criticized because of discontinuation of trolamine when grade 3 or 4 toxicities occurred. Pentoxifylline, oral zinc tablets have no significant benefits in acute skin reactions. Other issue about increased skin toxicity is usage of concomitant chemotherapy including cisplatin, 5-FU, taxans or EGFR inhibitors.
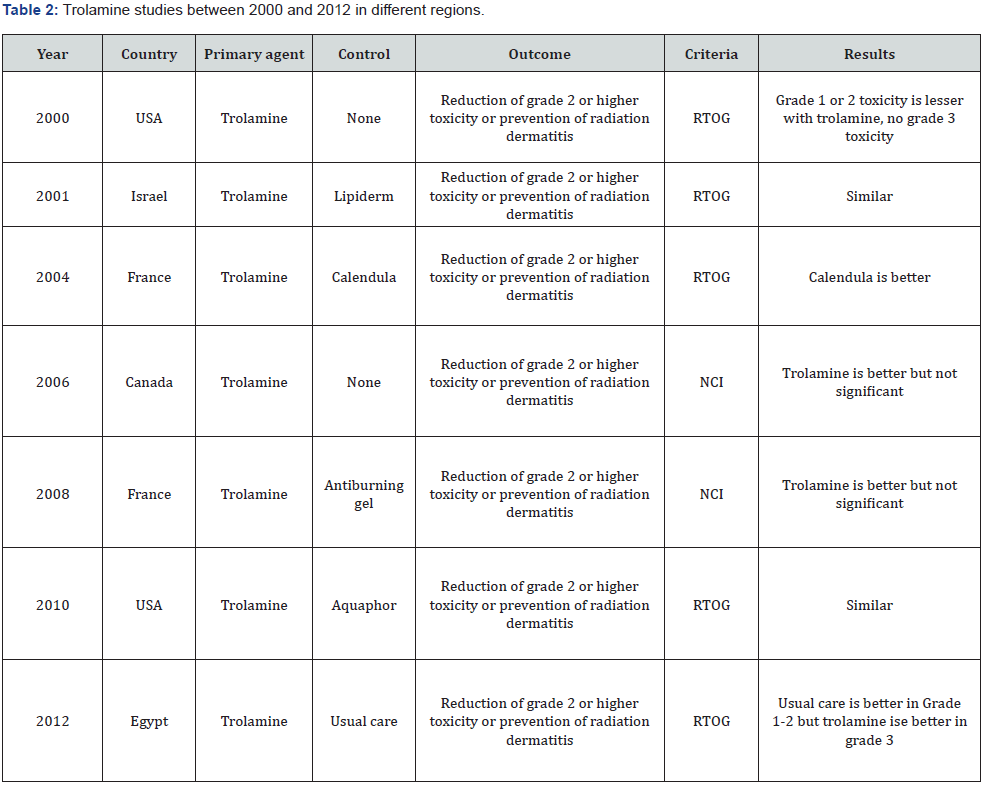
Unnecessary skin toxicity can be decreased with IMRT which allows minimal dose constraints of skin. Radiation oncologist should be in contact with medical oncologist, nurses and dermatologists to handle with radiation dermatitis and prevention of increased toxicity. Wound specialists or plastic surgery physician might be in team of radiation dermatitis management in case of adverse effects. Institutional policies about management of radiation dermatitis differ worldwide. Firstly, keeping the area clean and dry should be the first step. Gentle washing and drying of radiotherapy portal is so important associated with skin adverse effects. Which topical agent is superior over another is still unknown about radiation dermatitis. Chlorhexidine, hydrophilic dressing, hyaluronic acid, zinc oxid paste, silver sulvadiazine, beta glucan cream, drying gels are the alternative options for the management of dermatitis [6-11]. Studies done about trolamine betweeen 2000 and 2012 are listed in table 2. 2 studies resulted with similarity between groups. Calendula usage is better in only 1 study. 3 studies showed better results with trolamine and also 1 another resulted with better and lesser toxicity especially in grade 3 toxicity scale (Table 2).
Conclusion
We compared the initial usage of trolamine at the start of radiotherapy with second week of radiotherapy in breast cancer patients. The results were significantly better if this topical agent was used with the starting of therapy as a prophylaxis. We concluded that trolamine can be used in breast cancer or the cancers which skin is affected with radiotherapy. Trolamine is an effective and safe topical agent according to our results and literature. We think more randomized and multicenter studies are needed in future for the trolamine usage in cancer patients to prevent radiodermatitis.
References
- Meneses A, Reis P, Guerra E, Graziela De Luca Canto, Elaine Barros Ferreira et al. (2018) Use of trolamine to prevent and treat acute radiation dermatitis: a sytematic review and meta-analysis. Revista Latino Americana de enfermagem 26: 1518-1523.
- Hamza Abbas, René-Jean Bensadoun (2011) Trolamine emulsion fort he prevention of radiation dermatitis in patients with squamosus cell carcinoma of the head and neck. Supportive care in cancer 20(1): 185-190.
- Wang R, Wu F,Wang D, Liu Kai (2008) Clinical effect of biafine in preventing and treating radioactive skin destruction of nasopharyngeal carcinoma patients caused by concurrent intensity modulated radiotherapy and chemotherapy. Chin J Clin Oncol 5: 58-63.
- Del Rosso J, Bikowski J (2008) Trolamine containing topical emulsion: clinical applications in dermatology. Cutis 81(3): 209-214.
- Elliott EA, Wright JR, Swann RS, Felix Nguyen-Tân, Cristiane Takita, et al. (2006) Phase 3 trial of an emulsion containing trolamine for the prevention of radiation dermatitis in patients with advanced squamosus cell carcinoma of the head and neck: results of RTOG 99-13 trial. J Clin Oncol 24(13): 2092-2097.
- Rosenthal A, Israilevich R, Moy R (2019) Management of acute radiation dermatitis: a review of the literature and proposal for treatment algorithm. American Academy of Dermatology 81(2): 558-567.
- Cohen J, Jorizzo J, Kircik L (2007) Use of a topical emulsion for wound healing. J Support Oncol 5: 1-9.
- Walling H, Schulz K (2009) Trigeminal trophic syndrome: improvement with trolamine/sodium alginate containing topical emulsion. Journal of American Academy of Dermatology 61(1): 160-161.
- Kircik L (2009) Study of trolamine containing topical emulsion for wound healing after shave biopsy. Cutis 83(6): 326-332.
- Glesinger R, Cohen A, Berezovsky A, Yuval Krieger, Lior Rosenberg, et al. (2004) A randomized controlled trial of silver sulfadiazine, and saline soaked gauze in the treatment of superficial partial thickness burn wounds in pigs. Acad Emerg Med 11(4): 339-342.
- Lu S, Lu A, Paslin D (2020) A comparison of the wound healing efficacy of trolamine emulsion, manuka gel and polymyxin bacitracin ointment. Advances in skin & wound care 33(4): 217-220.






























