Molecular Oncology of Colorectal Cancers Prognosis and Prediction, Radiation Oncologist perspective!!
M Q Baig1 and Ansu Goel2*
1Associate Professor, Radiotherapy J K Cancer Institute, India
2Assistant professor, Radiotherapy J K Cancer Institute, India
Submission: July 21, 2022; Published: August 11, 2022
*Corresponding Address: Major M Q Baig, Associate Professor J K Cancer Institute, Kanpur, India
How to cite this article: M Q Baig, Ansu G. Molecular Oncology of Colorectal Cancers Prognosis and Prediction, Radiation Oncologist perspective!!. Canc Therapy & Oncol Int J. 2022; 22(1): 556080. DOI:10.19080/CTOIJ.2022.22.556080
Abstract
Colorectal cancers (CRC) are a common cancer globally. The commonest symptoms were rectal bleeding (57%), pain (44%), and altered bowel habits (26%). Thirteen percent of the patients had signet ring tumors. The CEA (Carcinoembryonic antigen) level estimation in patient serum commonly perform, surgery is a backbone of treatment unless until contraindicating factors are present after surgery tissue specimen required to send for not only histopathological studies rather also to look for tumor Immunohistochemistry studies in order to plan colorectal cancer very well directed chemotherapy according to its receptor as well as genetic or epigenetic alterations in BRAF, K-RAS, MMR , Most patients had localized or locally advanced disease. Twenty-eight percent of the patients had metastatic disease with liver being the commonest site of metastases (14%) followed by peritoneum and lung. More than half of the patients received treatment with a curative intent. Colorectal cancer in India differs from that described in the Western countries. We had more young patients, higher proportion of signet ring carcinomas, and more patients presenting with an advanced stage.
Keywords: Colorectal cancers; Immunohistochemistry; BRAF; K-RAS; MMR
Introduction
Colorectal cancer’’’, commonly known as ‘’’colon cancer’’’ or ‘’’bowel cancer’’’, is a cancer from uncontrolled cell growth in the Colon anatomy, Colon or rectum parts of the large intestine, or in the vermiform appendix, appendix Genetic analysis shows that essentially colon and rectal tumours are genetically the same cancer. Symptoms of colorectal cancer typically include Lower gastrointestinal bleeding rectal bleeding and anaemia which are sometimes associated with weight loss and changes in bowel habits. Genetics of colorectal cancer is the family history in two or more Degree of relationship first-degree relatives have a two to threefold greater risk of disease and this group accounts for about 20% of all cases. A number of genetic syndromes are also associated with higher rates of colorectal cancer. The most common of these is hereditary nonpolyposis colorectal cancer (HNPCC or Lynch syndrome) which is present in about 3% of people with colorectal cancer. Other syndromes that are strongly associated include: Gardner syndrome. Most deaths due to colon cancer are associated with metastatic disease. A gene that appears to contribute to the potential for metastatic disease – metastasis-associated in colon cancer-1 – has been isolated [1-4].
Greater than 75-95% of colon cancer may occurs in people with little or no genetic risk other risk factors include older age, male gender, high intake of fat, alcohol or red meat, obesity, smoking and a lack of physical exercise Approximately 10% of cases are linked to insufficient activity, In colorectal cancers mutational alteration However, by comparison, are frequent and affect hundreds of genes. For instance, there are types of small RNAs called micro RNAs that s are about 22 nucleotides long. These micro RNAs (or miRNAs) do not code for proteins, but they can “target” protein coding genes and reduce their expression. Expression of these miRNAs can be Epigenetics epigenetically altered. As one example, the epigenetic alteration consisting of CpG island methylation of the DNA sequence encoding miR-137 reduces its expression, and this is a frequent early epigenetic event in colorectal carcinogenesis, occurring in 81% of colon cancers and in 14% of the normal appearing colonic mucosa adjacent to the cancers. The altered adjacent tissues associated with these cancers are called Neoplasm Field defects in progression to cancer field defects [6-8].
Colorectal Cancer Genetics Epigenetic alterations are much more frequent in colon cancer than genetic causes Colorectal Cancer have increased β-catenin because of mutations in β-catenin (CTNNB1) that block its degradation, or they have mutation(s) in other genes with function analogous to APC such as AXIN1, AXIN2, TCF7L2, or Naked cuticle 1NKD Beyond the defects in the Wnt-APC-beta-catenin signaling pathway, other mutations must occur for the cell to become cancerous. The p53 protein, produced by the ‘’TP53’’ gene, normally monitors cell division and apoptosis kills cells if they have DNA pathway defects. Eventually, a cell line acquires a mutation in the ‘’TP53’’ gene and transforms the tissue from an adenoma into an invasive carcinoma. Other apoptotic proteins commonly deactivated in colorectal cancers are TGF-β and DCC Deleted in Colorectal Cancer. TGF-β has a deactivating mutation in at least half of colorectal cancers. Sometimes TGF-β is not deactivated, but a downstream protein named SMAD (protein) SMAD is commonly has Deletion (genetics)|deletion of its chromosome segment in colorectal cancer Some genes are oncogenes they are overexpressed in colorectal cancer. For example, genes encoding the proteins K RAS, C-Raf RAF, and PI3K, which normally stimulate the cell to divide in response to growth factors, can acquire mutations that result in over-activation of cell proliferation. The chronological order of mutations is sometimes important, with a primary KRAS mutation generally leading to a self-limiting. Hyperplastic or borderline lesion, but if occurring after a previous APC mutation it often progresses to cancer. A tumor suppressor, normally inhibits PI3K, but can sometimes become mutated and deactivated [9,10].
Discussion
Colorectal cancers divided on basis of immunohistochemistry reorts in three molecular subtypes on expression levels of EMT (Epethialial, Mesenchymal transition) these three molecular subtypes are , Epithelial subtype, Mesenchymal subtype, and Hybrid subtype. The epithelial subtype of colorectal cancers are E cadherine positive , Nuclear enzyme β Catenin positive but negative for vimentin these subtypes of colorectal cancers are having less propensity for regional lymphnode involvement as well as these tumor subtypes having low mitotic rate in view of these features these subtypes of colorectal cancers having good prognosis , However contrary to that Mesenchymal subtypes of colorectal cancers are negative for Ecadharin , negative for βcatenin but are positive for vimentin they shows higher rate of mitosis higher N/C ratio along higher propensity for regional lymphnode involvement henceforth shows poor prognosis.
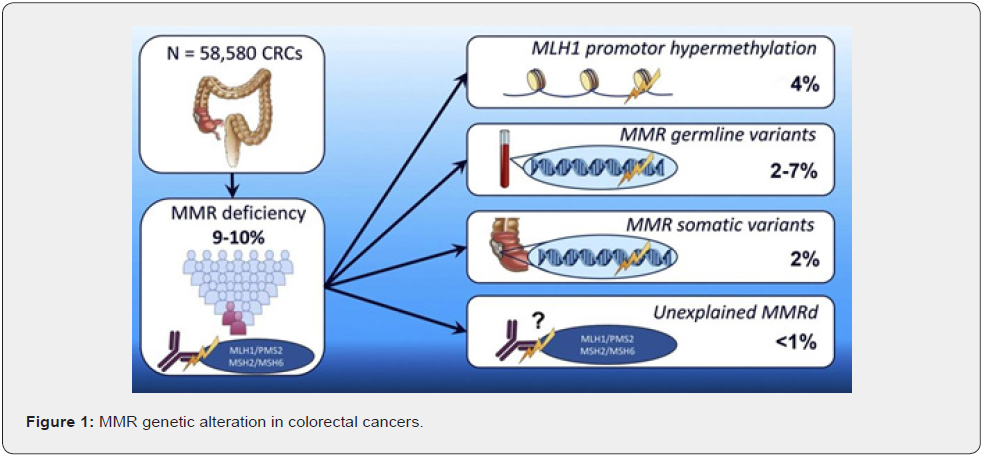
On Immunohistochemistry examination and studies, we look for these common genetic and epigenetic alterations these are MMR, BRAF, K-RAS mutations, MMR ( Mismatch repair ) proteins are a nuclear enzyme which participates in repair of base –base mismatch that occurs during DNA replications (among 9 to 10 percent colorectal cancer cases) refer to given below figure A during tumor cells proliferations. MMR genetic mutatations can occurs as as result of DNA hypermethylation, germline mutations (most common),or by somatic mutations and unknown reasons in very few cases (Figure 1).
BRAF mutations explain very poor prognosis of colorectal cancer patients median survival just limited up to one years of these colorectal cancer patients, BRAF is basically Protooncogene BRAF mutations have been found among 7 to 10 percent of colorectal cancers furthermore noticed BRAF mutations more commonly associated with right side of colonic cancers and less common with left side descending colonic cancers along with the exhibits higher rate of mitosis, higher N/C ratio , higher grade of histology , higher content of mucin in tumor along more commonly associated with peritoneal metastasis in view of all above features, makes the prognosis of BRAF mutant colorectal patients very poor outcome of disease. RAS, there are three Isoform of this gene in human out three K-RAS is being most commonly associated with many cancers in human K-RAS mutation found 17to 25 percent among different cancers but in case of Colorectal cancers K-RAS mutations with altered genes found in 30 to 40 percent cases, K-RAS mutation in colorectal cancers have been associated with poor survival and increased tumor aggressiveness.
Adjuvant Chemotherapy as per Molecular profile of colorectal cancer
Patient those having KRAS positive and having left sided colonic cancer ESMO suggests FOLFOX plus ANTI EGFR ( Cetuximab) provides good results, however if patients is KRAS positive but having right side of colonic cancer ESMO suggests FOLFIRI plus ANTI VEGF (Bevasuzimab) for good results, for metastatic colorectal cancer with KRAS positive wild type recommended chemotherapy is Cetuximab plus FOLFOX chemotherapy, BRAF Mutant patients recommended treatment is FOLFIRI plus ANTI VEGF.
Conclusion
The last half decade of CRC research has produced an important number of results. In order to personalize the treatment for CRC patients it is necessary to understand its natural history and malignant genesis mechanisms that help the disease progress. Unique biological signature of CRC can be distinguished by identifying biomarkers expression. Several markers have shown potential, Individualized approach studies based on particular disease characteristics will pave the way for personalized medicine. For each disease stage apart, therapeutic management based on biomarkers testing results will allow better use of health care resources and may relieve the patient of unworthy procedures.
Conflict of Interests
The authors declare that they have no conflict of interests.
References
- Tural D, Batur S, Erdamar S, Akar E, Kepil N, et al. (2014) Analysis of PTEN, BRAF and PI3K status for determination of benefit from cetuximab therapy in metastatic colorectal cancer patient’s refractory to chemotherapy with wild-type KRAS. Tumour Biol 35(2): 1041–1049.
- Thomaidis T, Maderer A, Formentini A, Bauer S, Trautmann M, et al. (2014) Proteins of the VEGFR and EGFR pathway as predictive markers for adjuvant treatment in patients with stage II/III colorectal cancer: results of the FOGT-4 trial. J Exp Clin Cancer Res 33(1): 83.
- Salmena L, Carracedo A, Pandolfi PP (2008) Tenets of PTEN tumor suppression. Cell 133(3): 403–414.
- Harris RC, Chung E, Coffey RJ (2003) EGF receptor ligands. Exp Cell Res 284(1): 2–13.
- Ryan D, Carberry S, Murphy AC, Lindner AU, Fay J, et al. (2016) Calnexin, an ER-induced protein, is a prognostic marker and potential therapeutic target in colorectal cancer. J Transl Med 14(1): 196.
- De Bruycker S, Vangestel C, Van den Wyngaert T, Wyffels L, Wouters A, et al. (2016) Baseline [(18)F]FMISO μPET as a predictive biomarker for response to HIF-1α inhibition combined with 5-FU chemotherapy in a human colorectal cancer xenograft model. Mol Imaging Biol 18(4): 606–616.
- Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, et al. (1996) Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 16(9): 4604–4613.
- Wang F, Zhang P, Shi C, Yang Y, Qin H (2012) Immunohistochemical detection of HSP27 and hnRNP K as prognostic and predictive biomarkers for colorectal cancer. Med Oncol 29(3): 1780–1788.
- Garde-Noguera J, Gil-Raga M, Evgenyeva E, Garcia JA, Llombart-Cussac A, et al. (2016) High pKDR immunohistochemical expression is an unfavourable prognostic biomarker in patients with advanced colorectal cancer treated with chemotherapy plus bevacizumab. Clin Transl Oncol 18(4): 405–412.
- Shim BY, Jung JH, Lee KM, Kim HJ, Hong SH, et al. (2013) Glucose transporter 1 (GLUT1) of anaerobic glycolysis as predictive and prognostic values in neoadjuvant chemoradiotherapy and laparoscopic surgery for locally advanced rectal cancer. Int J Colorectal Dis 28(3): 375–383.






























