The Binary Fronds-Phyllodes Tumour
Anubha Bajaj*
Department Hematology Unit, Faculty of Medicine, Alexandria University, Egypt
Submission: February 10, 2022; Published: March 16, 2022
*Corresponding Address: Anubha Bajaj, Histopathologist in A B Diagnostics, New Delhi, India
How to cite this article: Anubha B. The Binary Fronds-Phyllodes Tumour. Canc Therapy & Oncol Int J. 2022; 21(1): 556055. DOI:10.19080/CTOIJ.2022.21.556055
Preface
Phyllodes tumour of the breast is an infrequent fibro-epithelial neoplasm which represents as a morphologic continuum from benign breast tumours to malignant neoplasms. World Health Organization (WHO) subdivides phyllodes tumour into benign, borderline, and malignant categories contingent to nuclear atypia, stromal cellularity, mitotic activity, tumour perimeter and stromal overgrowth. The biphasic, fibro-epithelial lesion characteristically depicts a leaf-like epithelial component which is usually benign and tumour grading is pertinent to histology of stromal proliferation. Generally, associated chromosomal anomalies increase with enhancing tumour grade.
Phyllodes tumour is endowed with an inherent metastatic potential and tumour reoccurrence which pertains to histological grade. Majority of phyllodes tumours are benign and localized tumour reoccurrence is minimal. Apart from epithelial cells configuring ducts and lobules, phyllodes tumour is constituted of stromal, mesenchymal cells. Appropriate tumour diagnosis is obtained upon cogent histopathological assessment. Besides, alternative terminology of cystosarcoma phyllodes requires to be circumvented.
Disease Characteristics
Of obscure genetic profile, phyllodes tumour may concur with Li-Fraumeni syndrome. Exceptionally, phyllodes tumour occurring in male subjects appears due to hormonal imbalance and is associated with gynecomastia [1,2]. It is postulated that stromal induction of phyllodes tumour occurs on account of growth factors engendered by ductal epithelium of the breast. Infrequently, factors such as trauma, pregnancy, elevated oestrogenic activity, and lactation may stimulate tumour progression [1,2]. Possibly, endothelin-1 functions as a stimulator of breast fibroblasts and contributes to tumour growth [1,2]. Phyllodes tumour arises in middle aged females, generally between 4th to 5th decade. Phyllodes tumour constitutes an estimated 1% of breast neoplasia [1,2]. Benign phyllodes tumour is a commonly discerned variant. Although emerging within the breast parenchyma, ectopic breast tissue or vulva may demonstrate the neoplasm [1,2].
Disease Pathogenesis
Phyllodes tumour commences from extraneous connective tissue stroma of breast lobules and ducts wherein ligaments and mature adipose tissue circumscribing lobules, ducts, lymphatic, and vascular articulations may contribute to tumour emergence. Phyllodes tumour generally emerges de novo, although a fibro-adenoma may progress into phyllodes tumour [3,4]. The biphasic lesion exhibits significant epithelial- stromal interaction indicative of tumorigenesis. Absence of epithelial interaction within the stromal component may engender malignant metamorphosis [3,4]. Contemporary molecular classification of phyllodes tumour obtained with comparative genomic hybridization (CGH) exhibits reoccurring chromosomal imbalances, especially denominated as +1q, −6q, −13q, −9p, −10p or +5p. Phyllodes tumour displays repetitive mutations of MED12 and RARA genes [3,4]. Additionally, genomic mutations within cancer driver genes as NF1, RB1, PIK3CA, EGFR, TP53 and ERBB4 may be singularly associated with borderline and malignant phyllodes tumour. Chromosomal mutations within TERT promoter gene is common in borderline phyllodes tumour [3,4]. Besides, low grade and high grade (borderline or malignant) phyllodes tumour appear segregated into specific genetic groups pertaining to genomic alterations. Low grade phyllodes tumour is comprised of minimal or absent genetic modifications. High grade phyllodes tumour consistently exhibits chromosomal 1q gain and 13q loss [3,4]. Phyllodes tumour may exemplify foci simulating a well differentiated liposarcoma although genetic amplification or expression of CDK4 and MDM2 is absent [3,4]. Additionally, comparative genomic hybridization (CGH) can demonstrate interstitial deletion 9p21 along CDKN2A locus and 9p deletion within specific borderline and malignant phyllodes neoplasms [3,4].
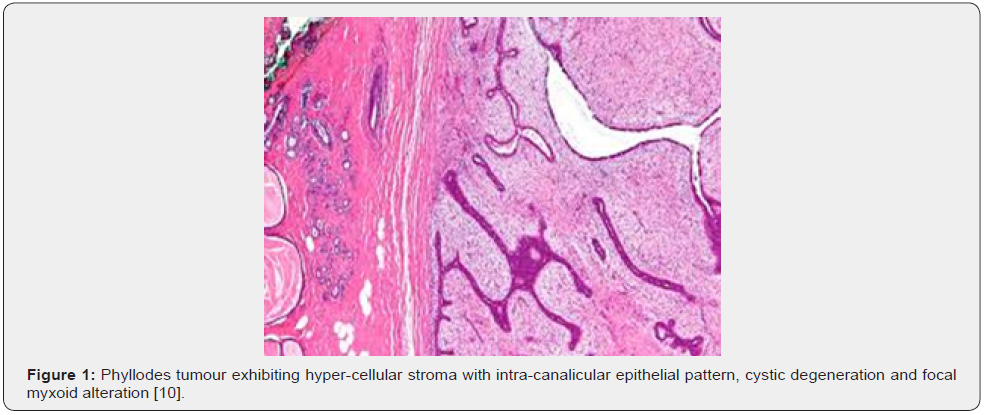
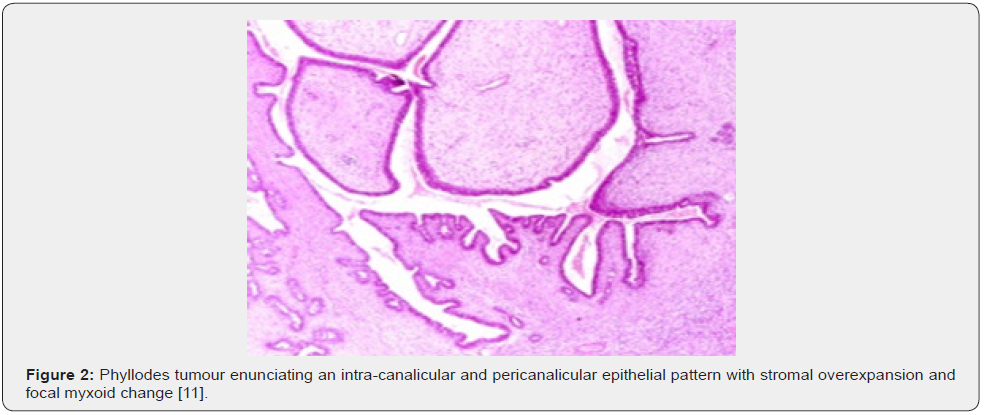
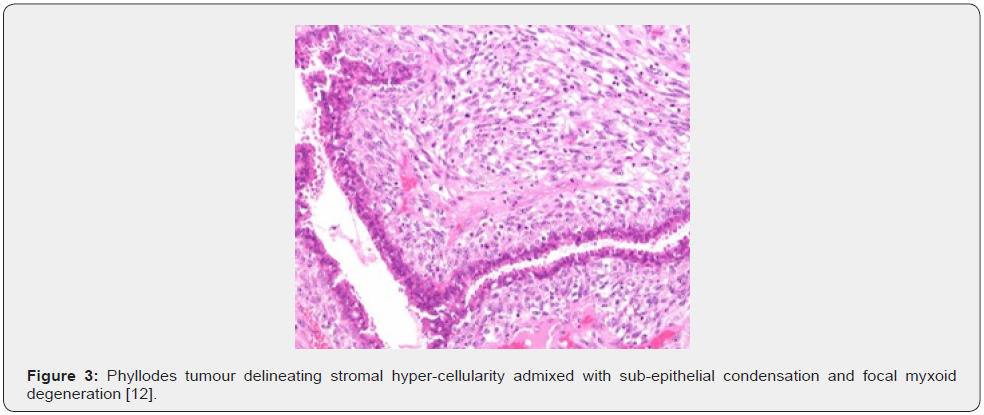
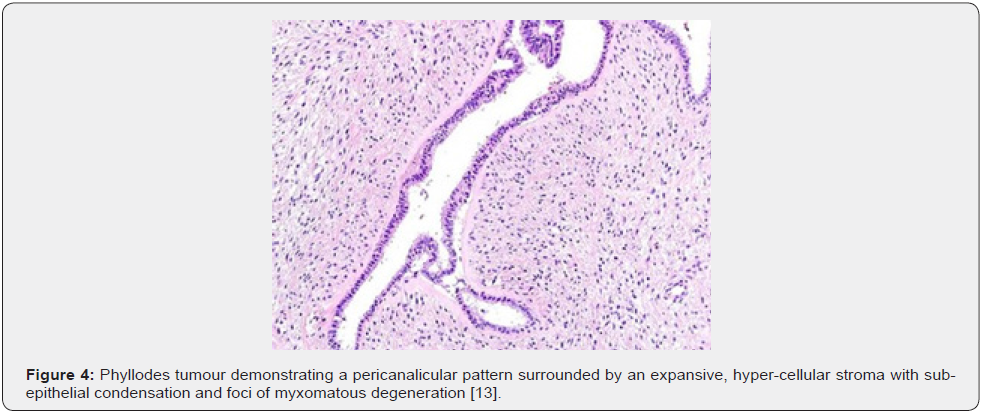
Clinical Elucidation
Phyllodes tumour manifests as a unilateral, firm, enlarging, painless, asymptomatic, mobile tumour nodule confined to breast parenchyma with distension of superimposed cutaneous surface and superficial veins. Tumour magnitude varies from one centimetre to 45 centimetres and the neoplasm may permeate entire breast parenchyma [5,6]. Blood-stained nipple discharge may ensue due to intra-ductal tumour elements or spontaneous infarction of the neoplasm. Ulceration and retraction of nipple are uncommonly discerned and may appear with enlarging, painful neoplasms [5,6]. Exceptionally, hypoglycaemia may occur due to secretion of insulin-like growth factor II [5,6]. Axillary lymph node enlargement is common although tumour metastases to regional lymph nodes is exceptional [5,6]. Generally, benign phyllodes tumour exhibits minimal localized tumour reoccurrence wherein recurrent neoplasms may be benign, borderline, or malignant. The neoplasm is devoid of distant metastasis or tumour-related mortality [5,6]. Borderline phyllodes tumour exemplifies an intermediate biological behaviour with enhanced possible localized tumour reoccurrence and infrequent distant metastasis [5,6]. Malignant phyllodes tumour enunciates significant localized tumour reoccurrence with distant tumour metastasis to pulmonary parenchyma, pleura, bone, central nervous system, diverse visceral organs, or soft tissue. Emergence of malignant heterologous elements, focal necrosis and tumour magnitude are factors which contribute to distant tumour metastasis. However, tumour metastasis into regional axillary lymph nodes is uncommon. Cellular accumulation within tumour perimeter is associated with localized tumour reoccurrence [5,6].
Histological Elucidation
Upon gross examination, phyllodes tumour configures a well circumscribed, firm, protrusive, spherical neoplasm with a shelled tumour margin [6,7]. Cut surface is fleshy, mucoid, bulging, whorled, bosselated, and tan, pink, or grey and delineates a leaflike pattern. Miniature lesions depict a homogeneous appearance. Enlarged lesions characteristically display a whorled pattern with curving clefts and leaf-like or budding structures. Foci of haemorrhage, focal necrosis, cutaneous ulceration, and cystic alterations are discerned [6,7].
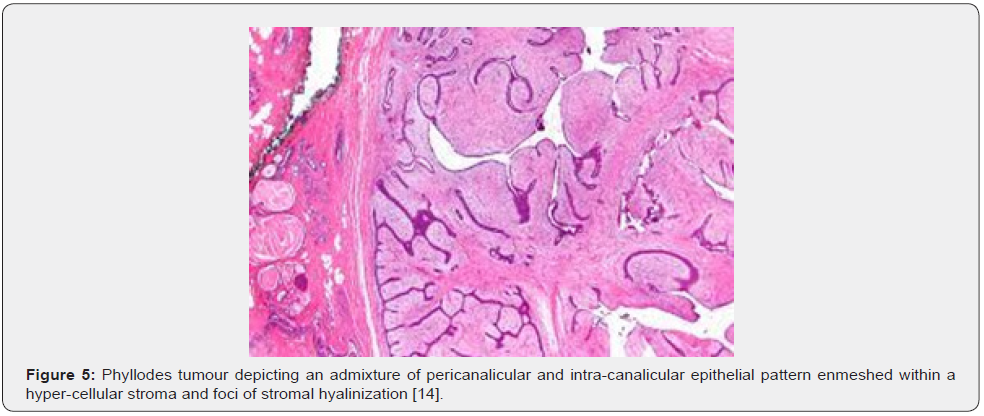
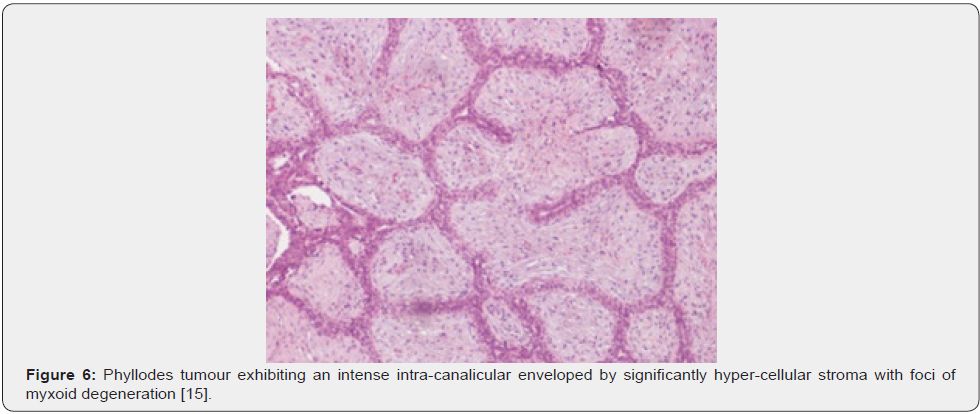
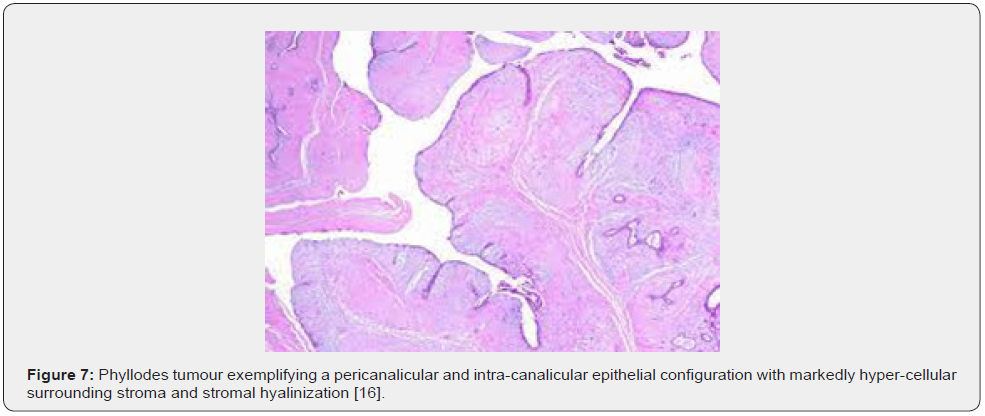
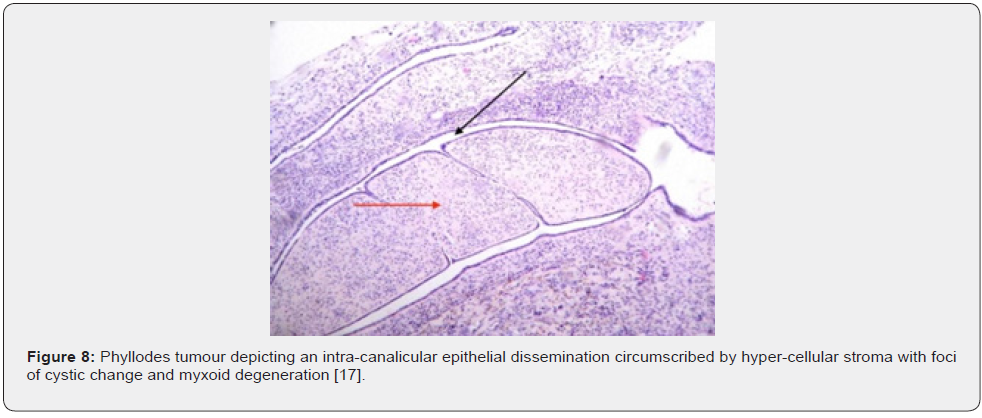
Nevertheless, ulceration and haemorrhage are non-indicative of malignant biological behaviour. Enlarged, infarcted benign phyllodes tumour may exhibit focal tumour necrosis [6,7]. Malignant neoplasms depict an infiltrative tumour border [6,7]. Upon microscopy, phyllodes tumour exemplifies an enhanced intra-canalicular epithelial pattern associated with leaf-like projections into circumscribing, variably distended, elongated cellular lumina [7,8]. Epithelial cell component is bland and comprised of layering luminal epithelial and myoepithelial cells which configure expansive, arc-like clefts superimposing fronds of enveloping stroma [7,8]. The leaf-like epithelial configuration occurs due to an expansive intra-canalicular pattern. Sub-epithelial condensation abutting the epithelium is observed. Enhanced cellularity of circumscribing connective tissue stroma ensues. Besides, cystic degeneration, haemorrhage, stromal hyalinization and myxoid modifications may ensue. Multinucleated, stromal giant cells are occasional. Myoepithelial layer is usually attenuated [7,8]. Upon cytology, clusters of fibromyxoid stroma are admixed with enlarged, wavy, and convoluted, benign epithelial cell aggregates. Epithelium-stroma proportion is decimated. Nuclear atypia and enhanced cellularity are observed in borderline and malignant phyllodes tumour. Occasionally, epithelial cell hyperplasia with enlarged, vesicular nuclei and miniature nucleoli may be delineated. Epithelial convolutions appear layered with fibroblasts. Multinucleated tumour giant cells and significant stromal anaplasia is observed in malignant phyllodes tumour. Contemporary grading of fibro-epithelial lesions as benign, borderline, or malignant phyllodes tumour is recommended as prognostic outcomes are concurrent with evolutionary disease spectrum [7,8].
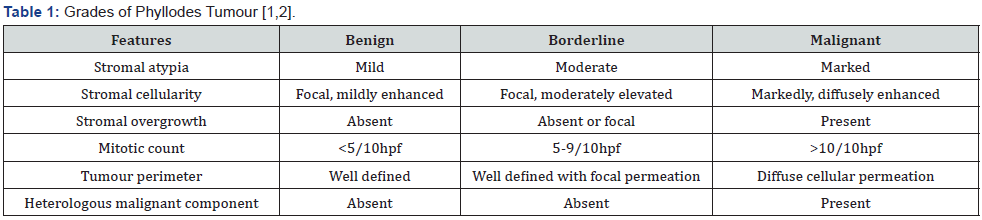
Benign phyllodes tumour manifests around ~75% of phyllodes tumour wherein the stroma is cellular, and nuclei of spindleshaped stromal cells appear regular. Mitoses is exceptional at below < 5 per 10 high power fields. Foci of stromal cellularity are sparse. Hyalinization or myxoid alterations may appear and indicate stromal heterogeneity. Tumour perimeter is well defined and pushing [7,8]. Borderline phyllodes tumour exhibits moderate stromal cellularity, circumscribed or focally invasive tumour perimeter, stromal atypia, and mitotic figures between 5 to 9 per 10 high power fields. Foci of stromal overgrowth are absent [7,8]. Malignant phyllodes tumour exemplifies a concurrence of significant nuclear pleomorphism within stromal cells, stromal overgrowth comprised singularly of stromal connective tissue with microscopic absence of epithelial elements within one lowpower field, enhanced mitotic figures ≥ 10 per 10 high power fields, augmented, diffuse stromal cellularity and an infiltrative tumour perimeter. Malignant phyllodes tumour with expansive stroma may depict minimal epithelial component, squamous metaplasia and incidental in situ or invasive squamous cell carcinoma [7,8].
Immunohistochemistry
Epithelial cells are immune reactive to cytokeratin, oestrogen receptors (ER), progesterone receptors (PR) and gross cystic disease fluid protein 15(GCDFP15). Stromal cells are immune reactive to vimentin, CD34, B cell lymphoma antigen 2 (BCL2) or oestrogen receptors beta (ERβ) [6,8]. Benign phyllodes tumour is minimally immune reactive to p53, proliferation marker Ki67, CD117, epidermal growth factor receptor (EGFR), p16 and vascular endothelial growth factor (VEGF). Aforesaid immune reactivity is contingent to histologic grade of phyllodes tumour. Particularly, p53 expression and Ki67 proliferation index appears to be associated with overall and disease- free survival [6,8]. Borderline or malignant lesions exhibit an elevated p53, proliferation index Ki67 and CD117. Focal immune reactivity to p63 can be discerned (6,8). Genetic expression of PAX3 and SIX1 is observed in borderline or malignant phyllodes tumour and is concordant with inferior clinical outcome [6,7]. Stromal cells are immune non-reactive to cytokeratin, p40 and p63 [6,7].
Differential Diagnosis
Benign phyllodes tumor requires a segregation from •which exhibits a combination of peri-canalicular and intra-canalicular pattern of epithelial cell dissemination. A leaf-like epithelial configuration is usually absent. Stroma is uniformly cellular and sub-epithelial condensation is generally non-discernible. Stromal cellularity may concur with mitotic figures, especially in cellular and juvenile fibroadenoma. Besides, stromal expansion is insignificant [1,2]. •fibromatosis is composed of spindle-shaped cells configuring elongated, sweeping fascicles. The tumefaction is devoid of an epithelial component. Tumour cells are immune non-reactive to CD34. β-catenin is localized within the nucleus [1,2]. Borderline or malignant phyllodes tumour requires distinction from •biphasic metaplastic carcinoma which enunciates an epithelial component with contributory in situ or invasive carcinomatous component. Additionally, the neoplasm may display malignant heterologous elements. Foci of epithelial differentiation are immune reactive to diverse epithelial antigens [1,2]. •monophasic metaplastic carcinoma is devoid of an epithelial component although malignant heterologous elements can be discerned. The neoplasm is immune reactive to cytokeratin and p63 [1,2]. •fibromatosis –like metaplastic carcinoma is devoid of significant cellular or nuclear atypia or stromal giant cells. Neoplasm is immune reactive to cytokeratin and p63. Nuclear accumulation of β-catenin is absent [1,2]. •primary or metastatic sarcoma is an infrequent neoplasm which lacks an epithelial component. Concurrence with adequate clinical history may be beneficial. However, malignant phyllodes tumour may simulate pure sarcoma of breast parenchyma [1,2]. •periductal stromal tumour may histologically simulate malignant phyllodes tumour. However, the non-circumscribed neoplasm is composed of proliferating spindle-shaped cells accumulated adjacent to open, tubular structures and lacks leaf-like articulations [1,2]. Also, well differentiated liposarcoma with mutation of MDM2 gene requires a distinction from phyllodes tumour [1,2].
Investigative Assay
Histological analysis is usually adequate for categorizing phyllodes tumour [8,9]. Imaging is contemplated to be imprecise wherein ultrasonography or mammography may be unsatisfactory in differentiating phyllodes tumour from fibro-adenoma [8,9]. Upon imaging, an elliptical, lobulated lesion is encountered with infrequent micro-calcifications [8,9]. Upon mammography, a characteristic, spherical, lobulated, dense tumefaction with circumscribed or focally indistinct tumour perimeter is observed. Although exceptional, enlarged foci of intra-tumoural calcification may occur [8,9]. Upon ultrasonography, phyllodes tumour represents as a hypoechoic, partly ill-defined or partially circumscribed tumefaction, frequently associated with posterior enhancement. Ultrasonography is considered inadequate for grading phyllodes tumour [8,9]. Upon colour Doppler, phyllodes tumour exhibits enhanced vascularity. Magnetic resonance imaging (MRI) can be adopted to assess infiltration of chest wall in malignant neoplasms. Characteristically, upon T1 weighted imaging, a heterogeneous, hypo-intense signal with focal haemorrhage is exhibited. T2 weighted imaging depicts a hyperintense, lobulated mass. Slit-like spaces appear fluid filled, and circumscribing tissue may be hyper-intense [8,9].
Therapeutic Options
Comprehensive surgical excision with eradication of a significant perimeter of uninvolved normal tissue exceeding > one centimetre magnitude is recommended and usually curative. Aforesaid procedure decimates possible localized tumour reoccurrence. Enlarged tumours can be subjected to mastectomy [8,9]. Additionally, localized tumour excision with a clear tumour perimeter may be adopted. Benign phyllodes tumour can be subjected to surgical extermination with removal of a narrow tissue margin. Although devoid of consensus, eradication of 10 millimetre of circumscribing soft tissue is contemplated to be adequate [8,9]. Localized tumour reoccurrence is elevated following breast-conserving surgical procedures although metastasis-free survival or overall survival remain consistent and appear identical with mastectomy [8,9]. Adjuvant radiation therapy may decimate localized tumour reoccurrence. Results of radiotherapy may be ambiguous wherein localized tumour progression may be curtailed although overall survival of borderline and malignant phyllodes tumour remains unaltered [8,9]. Adjuvant chemotherapy appears non beneficial [8,9]. Generally, dissection of axillary lymph nodes is unnecessary [8,9]. Phyllodes tumour is accompanied by a favourable prognosis. Tumour reoccurrence is contingent to tumour magnitude and surgical procedure adopted and usually occur within ~ 3 years [8,9]. Malignant phyllodes tumour is associated with an inferior prognosis. Distant metastasis of malignant neoplasms is documented and may appear within pulmonary or hepatic parenchyma, bones, brain, or cardiac muscle [8,9].
Stromal overgrowth-absence of epithelial elements with singularly disseminated stroma within one low power field. Heterologous malignant elements are composed of chondroid, lipomatous, bone, skeletal muscle, smooth muscle, or vascular articulations.
References
- Limaiem F, Kashyap S (2021) Phyllodes Tumour of The Breast. Stat Pearls International, 2021 Treasure Island, Florida, USA.
- Zhang Y, Kleer CG (2016) Phyllodes Tumor of the Breast: Histopathologic Features, Differential Diagnosis, and Molecular/Genetic Updates. Arch Pathol Lab Med 140(7): 665-671.
- Jing P, Wei B, Xiaoqin Yang (2018) Phyllodes tumor of the breast with nipple discharge: A case report” Medicine (Baltimore) 97(52): e13767.
- Adesoye T, Neuman HB (2016) Current Trends in the Management of Phyllodes Tumors of the Breast” Ann Surg Oncol 23(10): 3199-3205.
- Mitus JW, Blecharz P, J Jakubowicz, M Reinfuss , T Walasek (2019) Phyllodes tumors of the breast. The treatment results for 340 patients from a single cancer centre. Breast 43: 85-90.
- Tan WJ, Thike AA, Boon Huat Bay, Puay Hoon Tan (2014) Immunohistochemical expression of homeoproteins Six1 and Pax3 in breast phyllodes tumours correlates with histological grade and clinical outcome. Histopathology 64(6): 807-817.
- Ruiz-Flores L, Ebuoma LO, Marcelo F Benveniste, Chandandeep Nagi, Tamara Ortiz Perez, et al. (2018) Case Report: Metastatic Phyllodes Tumor. Semin Ultrasound CT MR 39(1): 122-126.
- Yasir S, Nassar A, Rafael E Jimenez, Sarah M Jenkins, Lynn C Hartmann, et al. (2018) Cellular fibroepithelial lesions of the breast: A long term follow up study. Ann Diagn Pathol 35: 85-91.
- Goto W, Kashiwagi S, Koji Takada, Yuka Asano, Tamami Morisaki, et al. (2018) A Case of a Malignant Phyllodes Tumor That Was Difficult to Distinguish from Stromal Sarcoma” Gan To Kagaku Ryoho 45(13): 2429-2431.
- Image 1 Courtesy: Wikimedia commons
- Image 2 Courtesy: Pathology outlines
- Image 3 Courtesy: Wikiversity.com
- Image 4 Courtesy: Flickr.com
- Image 5 Courtesy: Wikipedia
- Image 6 Courtesy: Pinterest
- Image 7 Courtesy: Stat Pearls
- Image 8 Courtesy: Medbullets.com






























