Conceptual framework of Modern Molecular Oncology Paving the ways for Advance Targeted Therapies for Cancer Treatments
Mirza Qaiser Baig*
Associate professor, J K Cancer Institute Utter Pradesh Govt, India
Submission: February 09, 2022; Published: February 24, 2022
*Corresponding Address: Mirza Qaiser Baig, Associate professor, J K Cancer Institute Utter Pradesh Govt, India
How to cite this article: Mirza Q B. Conceptual framework of Modern Molecular Oncology Paving the ways for Advance Targeted Therapies for Cancer Treatments. Canc Therapy & Oncol Int J. 2022; 21(1): 556052. DOI:10.19080/CTOIJ.2022.21.556052
Abstract
Cancer cells are those cells that overcome the boundaries of impending unrestricted divisions and will multiply and further sustain aberrations that’s fuel its growth, survival, invasion and migration (metastasis) to establish its presence in distant organs. Technologies have emerged that facilitate understanding of pathways which got disturbed leading the cause of cancer genesis it also opens the gate for targeted therapies that targets specific molecules in the pathways of cancer genesis and its further divisions like few examples , Gleeve which inhibits a subset of tyrosin kinase is approved for the treatment of CML,GIST tumors , Irresa which inhibits EGFR used in NSCLC, Herceptin an monoclonal antibody used in her-2 neu positive breast cancer treatment , Valcade , a Proteosome inhibitor that elicits programmed cell death is approved for the treatment of Multiple myeloma treatment. many more drugs are currently under trails.
Keywords: Targeted therapies; Metastasis; Migrations
Discussion
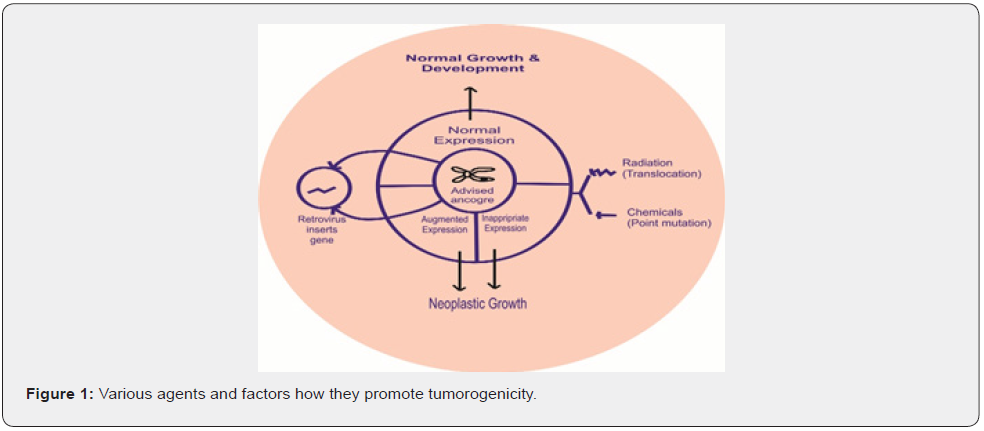
Historical backgrounds Molecular basis of cancer development came from study of viruses; the first cancer causing virus was ROUS SARCOMA VIRUS –RSV, was discovered by PEYTON ROUS in 1911 for that he was given Nobel Prize in 1966. Molecular basis of RSV causing cancer found that virus src oncogene, virus src is a homolog of proto-oncogene [1-3]. In early 1980 Ha-RAS and K-RAS genes were discovered in the foci resulting from transfection of immortalized mouse fibroblast with human cancer cell line DNAs, Another avenue for cancer gene discovery was development of CYTOGENETIC study here translocation study leads to the discovery of fusion genes that codes oncogene proteins example BCR-ABL oncogene develop by the translocation of chromosomes 9 and 22 and frequently present in CML. RBI retinoblastoma gene was discovered in 1986 in sporadic or familial retinoblastoma (Figure 1).
Molecular Basis of Cancer
Research and study on gene restriction fragment length, polymorphism based positional cloning tremendous technological advancement and concepts about pathways and mechanisms that lead to cancer formation have been explored. As a result now human genomic study gone up to unprecedented level , Genomic sequencing study can provide vital information and help in defining “MOLECULAR SIGNATURES” associated with specific types of cancer .till now 25000 human genes sequencing have been finalized and studied along with their DNA sequencing representing specific gene that is the most promising part of study that stratifies tumor based specific gene that is called as MOLECULAR SIGNATURES in several ways these molecular signatures predicts prognosis and treatment outcome of specific cancer . The advent of highly sophisticated microscopy and image analysis tools has facilitated thousands of small molecules and genes that can be identified that modulate specific type of cancer, induction of Apoptosis, migration of tumor cells and its critical pathways and thus potential drugs that can target specific cancer can be made [4].
Cancer Genes
Gain of function of Oncogene and loss of functions of TSG Tumor suppressor genes results in the development of cancer as per the data more than 1% of genes (that means 291 out of total estimated genes of 5000 in numbers) may contribute to the development of cancer.70% of above mentioned genes are affected by somatic mutations, 20 % of above mentioned genes are affected by Germ line mutations, 10% of above mentioned genes are affected by both of above mutations. Dominate mutations comprises 90% of somatic mutations and out of these 70% are associated with translocation (in haemopoeitic/ mesenchymal malignancies). 90% of germ line mutations in familial cancer syndromes are in tumor suppresser genes because most dominate gene would cause lethal effect during development and would result in cell death. Apart from oncogenes there are many other genes act as modifiers and do have impact on cancer cells behavior that’s why some smokers never develop lung cancer and some patients never develop metastasis, or some patients respond to cancer chemotherapy and some patients do not [5].
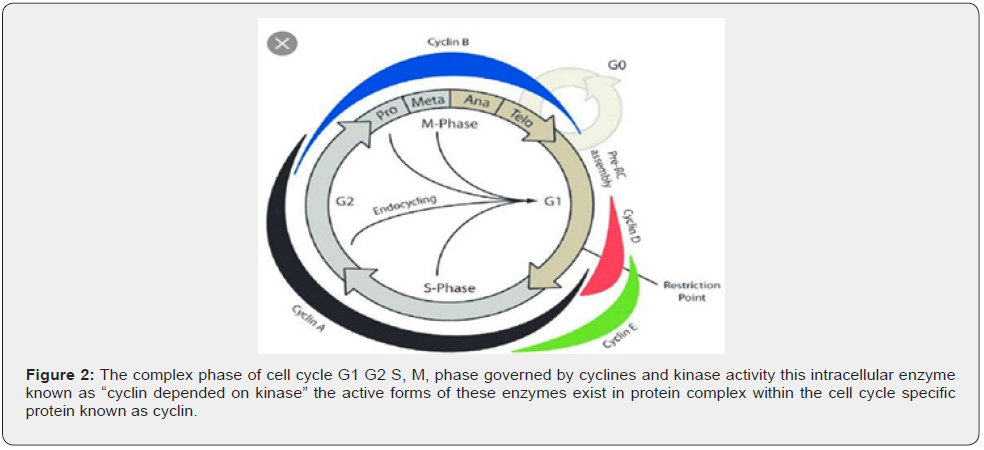
Cell Cycle Regulation
Cell cycle having different phases like G0, G1, S, G2M cell cycle regulated by cycline dependent kinas family of enzymes, cycline are synthesized at beginning of phase and destroyed at the end of specific phase of cell cycle (Figure 2).
Cellular Apoptosis and Autophagy
During cell division DNA damage or mutations occurs and process of cell death initiated , apoptosis regulated by TNF receptors proteins other pathways is cell signaling, the mitochondrial mediated pathway involves the release of Cytochrome-C from mitochondrial membrane this process is regulated by gene BCL-2 ( Antiapoptotic gene ) inhibition of BCL- 2 leads to activation of P53 gene which in turn leads to apoptosis , apoptosis is the only process involve in cell / tumor cell growth arrest there is another process named AUTOPHAGY also works in this process cell destructions happens when cell suffers nutrient starvation cell digest their own intracellular organelle the cytoplasmic LYSOSOME gene involve in autophagy are BECLINE-1 TUMOR SUPPRESSER GENE , induction of Autophagy start by nutrients sensing m-TOR kinas [6].
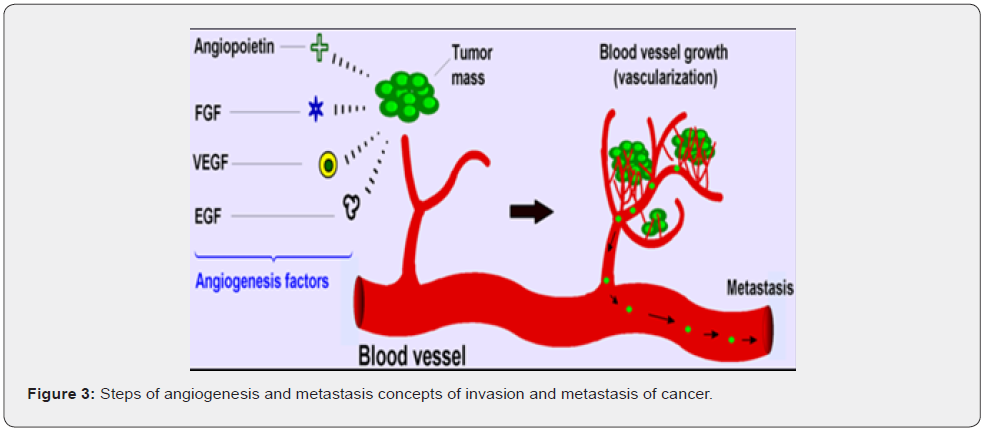
Angiogenesis
Tumor tissues requires an adequate supply of oxygen and nutrients as we as effective way to remove waste products thus tumor gaining access to host vascular system and formation of tumor blood supply are considered as rate limiting steps in tumor progression. Induction of angiogenesis depends upon anti and proangiogenic factors which are release by cancer cells an important member of genes responsible for these factors are VEGFR factors apart from VEGFR other factors are, IL8, IL6, ANGIOPOETINE, PLATLET DERIVED GROWTH FACTORS all these factors served to promote formation of new blood vessels for tumor tissues (Figure 3). Only 10% cancer patient’s death occurs due to primary cancers, 90% death occurs by secondary or metastasis of primary cancers cancer spreads by two ways.
A-Invasion, B- Metastasis
Invasion refers to penetration of cancer cells to neighboring tissues, metastasis refers to ability of cancer cells to penetrate blood/lymphatic channels and reach distant organs, molecular mechanism of metastasis involve two process or events first is cell to cell second is cell to matrix adhesion and disintegration of extracellular matrix. Cell to cell adhesions molecules is given below
A- INTEGRINES- This is responsible for adhesions of cells and deadhesions of cells from extracellular matrix for that integrines send signals from outside of cells to inside of cells to do so.
B- CADHERINES – This helps in adhesions of cells by destructions of extracellular matrix, loss of E-CADHERINES results in invasion potential of tumor cells.
MATRIX DEGRATION – extracellular matrix provides the fundamental support for cell migration and prevents induction of apoptosis, remolding of tumor extracellular matrix via proteolysis is necessary step in cancer cell metastasis.
There are three principal enzymes that does degradation of extracellular matrix and promotes invasion of cancer above mentioned three enzymes are given below
A- MATRIX METALLOPROTENASES, MMPS – expression of this enzyme in tumor cells promotes COLLAGENASE, GELATINASES which promotes extracellular matrix degradation which facilitates migration of cancer cells via degraded extracellular matrix.
B- TISSUE SERINE PROTEASES – these are urokinase plasminogen activating enzymes they act by doing degradation of plasminogen into plasmin that in turn leads to degradation of extracellular matrix.
C- HEPARANASE – increase heparanase activity leads to degradation of heparins sulphate as well as extracellular matrix degradation which lead the migration of cancer cells.
Conclusion
In view of achieving early diagnosis of cancer as well as designing targeted therapies of a specific cancer we need to understand tumor radiobiology as well as tumor molecular oncology in depth with accuracy. As exosomes express specific markers on their surface, they are the ideal candidates that could lead to more efficient management of cancer.
Conflict of Interest
The author declares no conflict of interest.
References
- Perkins ND (2003) Oncogenes, tumor suppressors and p52 NF-B. Oncogene 22(48): 7553–7556.
- Lin L, DeMartino GN, Greene WC (1998) Cotranslational biogenesis of NF-B p50 by the 26S proteasome. Cell 92(6): 819-828.
- Cohen S, Achbert-Weiner H, Ciechanover A (2004) Dual effects of Bkinase mediated phosphorylation on p105 fate: SCF(TrCP)-dependent degradation and SCF(TrCP)-independent processing. Mol Cell Biol 24(1): 475–486.
- Druker Brian J, M Talpaz, D J Resta, B Peng, E Buchdunger et al. (2001) Efficacy and Safety of a Specific Inhibitor of the BCR-ABLTyrosine Kinase in Chronic Myeloid Leukemia. N Engl J Med 344(14): 1031–1037.
- Goldman, John M, Junia V Melo (2001) Targeting the BCR-ABL Tyrosine Kinase in Chronic Myeloid Leukemia. N Engl J Med 344(14): 1084–1086.
- Nowell P, J Rowley, A Knudson (1998) Cancer Genetics, Cytogenetics—Defining the Enemy Within. Nature Medicine 4(10): 1107–1114.






























