The Perfect Combination of the Most Powerful Cancer Immunotherapy with the Best Targeted Chemotherapy
Vladimir N Pak*
Freelance Researcher, Toronto, Canada
Submission: February 11, 2022; Published: February 18, 2022
*Corresponding Address: Vladimir N Pak, Freelance Researcher, Toronto, Canada
How to cite this article: Vladimir N P. The Perfect Combination of the Most Powerful Cancer Immunotherapy with the Best Targeted Chemotherapy. Canc Therapy & Oncol Int J. 2022; 20(5): 556050. DOI:10.19080/CTOIJ.2022.20.556050
Abstract
Cancer is one of the leading causes of death globally. There are cancer treatments like chemotherapy, radiotherapy, surgery, and immunotherapy. A combination of therapies can lead to a cancer cure. Myeloid-derived suppressor cells (MDSCs) activity prevents the success of many immune- and chemotherapies. MDSCs depletion prevails over other cancer immunotherapies because it activates both innate and adaptive immunity. On the other hand, the best-targeted chemotherapy specifically kills cancer cells, including cancer stem ones that are at the roots of metastases leading to 90% of deaths of cancer. The low differentiated MDSCs, cancer stem cells, and most cancers absorb alpha-fetoprotein (AFP) loaded with nutrients through AFP receptor (AFPR)-mediated endocytosis. So, the AFP-toxin drug is targeted chemotherapy that hits simultaneously to MDSCs and cancer cells. In my opinion, it is the perfect combination of the most powerful cancer immunotherapy and the best-targeted chemotherapy. For example, the AFP-maytansine conjugate demonstrated 100% survival in tumor-bearing T cell-deficient mice.
Keywords: Cancer; Immunotherapy; Targeted chemotherapy; Alpha-fetoprotein receptor; Tumor microenvironment; Myeloid-derived suppressor cell; Natural killer cell
Abbreviations: AFP: Alpha-fetoprotein; AFPR-Alpha-fetoprotein receptor; MDSC-Myeloid-Derived Suppressor Cell; TME-Tumor Microenvironment; NK: Natural killer
Introduction
There are several hallmarks of cancer that open new dimensions in treatments [1]. Nevertheless, some of them can be considered “more equal than others”. In most cancer cells, apoptosis is disabled. In addition, the immune system in cancer patients is tolerable to the disease. The rational way to treat cancer is to repair apoptosis in cancer cells and cancel the immune tolerance in cancer patients. Apoptosis can be restored by the toxin precise delivery inside the cancer cell or targeted chemotherapy, while immune response – by the powerful immunotherapy. The combination of two can lead to cancer cure.
Discussion
Apoptosis erases the cells with unrepairable mutations. Irradiation and some chemotherapies are used to generate those mutations in cancer cells. They expect the consequent p53-dependent apoptosis. Unfortunately, the main tumor suppressor protein p53 is disabled in human cancer cells [2]. That is why irradiation and chemotherapy can fail. Nevertheless, “healthy” apoptosis is very effective against cancer. For example, elephants due to extra copies of the p53 gene (and consequently permanently active p53) have less than 5% cancer mortality rate compared to humans 11-25% [3]. The mitochondrion follows the p53 and is known as “a point of no return” in the intrinsic apoptosis pathway. So, toxins hitting mitochondrion or other targets independent of p53 should be effective in killing cancer cells [4]. Neither DNA mutations, p53 condition, multiple drug resistance of cancer cells, nor will dependence of cell cycle phase prevent the cell death.
The second reason for therapies inefficacy or failure is the tumor microenvironment (TME) that protects tumor cells from both innate and adaptive immunity attack. TME is generated by myeloid-derived suppressor cells (MDSCs) that are a small subpopulation of the low differentiated monocytes involved with the physiological regulation of the immune response during pregnancy and cancer [5,6]. MDSCs contribute to maternal-fetal tolerance or suppressing the immune attack to cancer cells [7].
Dr. Govallo discovered the similarity between suppression of the immune system during pregnancy and cancer [8]. The suppression of the immune response in pregnancy is local and strong. The embryo is an “alien” to the mother because of the father’s genes and proteins and should be rejected. Nevertheless, even surrogate motherhood with no genetic relationship to embryos is possible. During pregnancy, an embryo generates oncofetal proteins. Oncofetal proteins are expressed by embryo cells during fetal development and next time during cancer (onco) development. Oncofetal proteins act through the mother’s immune cells, and together with hormones neutralize antiembryo immune response leading to immune tolerance [9]. Cancer and pregnancy use the same mechanisms to suppress the immune system in the most effective and powerful way by using oncofetal proteins [10]. Dr. Govallo extracted proteins from a human placenta (that belongs to the embryo) and has shown that the extract effectively blocks white blood cells activity. He used the extract to vaccinate 35 previously incurable cancer patients with metastases of different cancers. Vaccination reversed the immune suppression and led to remarkable results: quantitative reduction of the tumors. A 5-year survival rate of 77.1% and a 10- year survival rate of 65.4% were demonstrated [8].
Oncofetal proteins suppress the immune system during pregnancy and cancer through powerful MDSCs and T regulatory cells [7,11]. MDSCs are the subpopulation of low differentiated monocytes, they migrate from the bone marrow and together with the regulatory T cells create the TME that prevents the cytotoxic activity of the innate and adaptive cells against tumor cells. Neutrophils that play a significant role in cancer biology and the innate immune system have been overlooked as immunologists focused on T and B cells of the adaptive immune system. Nevertheless, the innate immune system and natural killer (NK) cells are crucial in cancer prevention. NK cells are famous for their defense against tumors. Like cytotoxic T cells, they kill cells with granzyme B that directly activates caspases and apoptosis. Neither p53 conditions, multiple drug resistance, nor dependence on the cell cycle can prevent the inevitable apoptosis of cancer cells. NK cells destroy low-differentiated cancer stem cells and play a critical role in the early prevention of the spread of tumors and metastases [12] which is the leading cause of 90% of cancerrelated deaths. NK cells, like other immune cells, are suppressed by the TME directly and/or indirectly.
To unleash an immune defense army of NK, T, and B cells, macrophages, neutrophils, and other cells to attack cancer one needs to destroy MDSCs. MDSCs death cancels the TME and activates both innate and adaptive immunity attacks on cancer cells [13]. MDSCs and NK cells can be targeted in the TME for therapeutic purposes [13-15]. The discovery of alpha-fetoprotein (AFP) receptor (AFPR) on MDSCs by Dr. Belyaev and co-workers is a breakthrough [16]. AFPR became a new valuable marker of MDSCs which are present in healthy people and are accumulated in people with disease [17]. Dr. Belyaev and co-workers used AFPdaunorubicin conjugate to specifically target and deplete MDSCs, cancel immune suppression, and reduce tumors in the mice models [16].
The AFP functions and clinical application are covered well in [18,19]. AFP is the main oncofetal protein produced by the embryo that serves two critical functions: first, it transports nutrients from the maternal bloodstream into rapidly growing embryonic cells through AFPR-mediated endocytosis. For example, the omega-3 polyunsaturated fatty acid is an essential nutrient that the mother must take by food as she does not produce it herself. AFP captures omega-3 from albumin, crosses the placenta which typically separates the two circulations, and delivers the nutrients into the AFPR-positive embryonic cells.
The second AFP function is immune regulation [18]. AFP normalizes immune system responses, so the mother’s immune system doesn’t attack the embryo. The same AFP function can be observed in adults. AFP’s physiological role in adults is, throughout its interaction with AFPRs, to regulate and facilitate the entry of omega-3 fatty acids into living cells undergoing growth and differentiation. Dr. Suzuki and co-workers isolated and characterized a specific AFPR almost exclusively on human peripheral monocytes that are involved with the physiological regulation of the immune response [20]. Dr. Esteban and coworkers have shown that an AFP-AFPR autocrine system might operate in human normal and malignant blood monocytes [21].
Unlike AFP, which was the first clinical oncomarker discovered by Dr. Abelev and Dr. Tatarinov, AFPR should be considered the #1 oncomarker. AFPR is not universal oncomarker, but it was found in most liquid and solid cancers [22-24]. Due to the AFPR absence in healthy cells, AFP have been used for the toxins targeted delivery to cancer cells [18]. So, the AFP-daunorubicin conjugate used by Dr. Belyaev naturally combined cancer immunotherapy and targeted chemotherapy. The high efficacy of the low doses of AFP-toxin injectable and peroral preparations in cancer patients’ treatments supports the idea of the dualism of AFP-toxin action [4].
As can be seen in Figure 1, AFP-toxin drug immunotherapy action prevails chemotherapy one. The more likely and powerful immunotherapy than targeted chemotherapy action is proved by the experiments with peroral administration of porcine AFPatractyloside complex [25]. Atractyloside hits the mitochondrion leading to p53-independent apoptosis of the targeted cell. Neither AFP, nor the toxin, were detected in the blood, they could not directly hit MDSCs or cancer cells, but they hit other AFPbinding immune regulatory cells in the gut lymphatic nodes that eventually led to liver metastases reduction. The mechanism of AFP-atractyloside and other peroral AFP-toxin complexes’ actions is to be discovered yet [4].
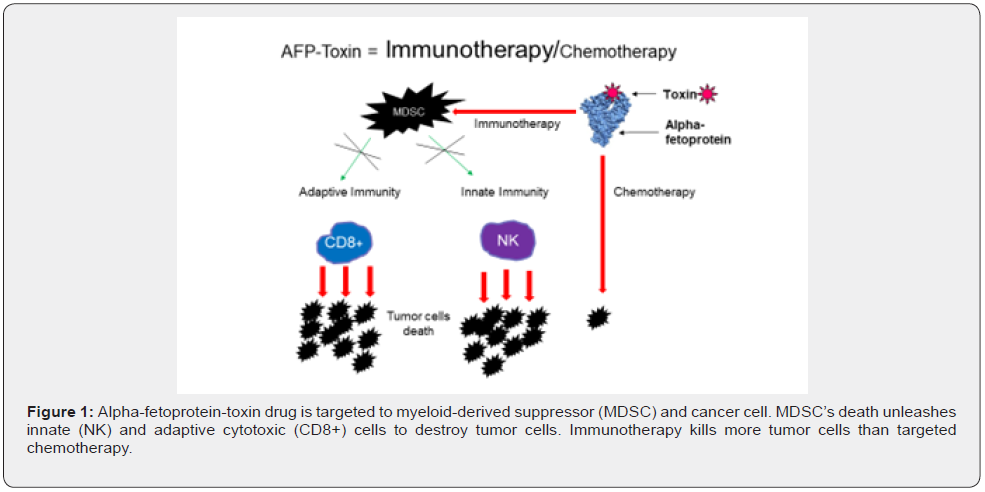
AFP-toxin drugs can be used as non-covalent complexes mentioned earlier or as chemical conjugates [4,18]. Attaching a chemotherapy payload to AFP by covalent link provides the same selective delivery of chemotherapy to cancer cells and immune suppressor cells while bypassing normal cells. This results in increased efficacy by targeting and killing cancer cells and immune suppressor cells. Thus, the AFP-maytansine (the toxin more potent than paclitaxel) conjugate has demonstrated a statistically significant reduction in tumor volume in mice compared to control groups. The tumor reduction continued following treatment discontinuation with tumor volumes falling below the limit of detection in 9 of 10 animals. There was a 100% survival in the AFP-maytansine group at day 60, compared to 0% survival in the control group by day 38 [26]. It should be noted that athymic nude (NCr-nu/nu) mice used in the experiment were T cell-deficient and hence, the antitumor effect was due to combined action of the innate immunity unleashed by MDSCs depletion (and NK cells in particular) and targeted chemotherapy. The effect is expected to be enhanced in humans without adaptive immunity deficits. So, AFP-toxin combines the most powerful cancer immunotherapy and the best targeted chemotherapy.
Conclusion
Apoptosis and the immune system protect most of us from cancer. To restore these defense mechanisms broken in cancer patients one needs to rely on both protective mechanisms and combine the two cancer therapies for this purpose. The targeted chemotherapy restores apoptosis inside the cancer cells, while immunotherapy will kill them from outside. The preferable targeted chemotherapy should induce apoptosis in cancer cells with efficacy like in elephants, and immunotherapy should reverse the immune suppression and generate not temporary but the durable anticancer response with efficacy like in Dr. Govallo’s experiments. AFP-toxin drugs serve as targeted chemotherapy to the regulatory suppressor cells and cancer cells at the same time. MDSC-targeted immunotherapy prevails cancer cells-targeted chemotherapy action because it reverses the TME. AFP-toxin drug is the perfect combination of the most powerful cancer immunotherapy with the best targeted chemotherapy. This combination provides a simple, applicable strategy to simultaneously increase the potency and safety of toxins, decreasing systemic dissemination relative to their parent compounds, increasing immunotherapy and chemotherapy, and enhancing anti-tumor efficacy while greatly reducing systemic toxicity, and it forms the memory. In addition, the AFP-toxin therapy is not personalized which makes it cheap.
Conflicts of Interest
No Conflicts of Interest.
References
- Hanahan D (2022) Hallmarks of Cancer: New Dimensions. Cancer Discov 12(1): 31-46.
- Vogelstein B, Sur S, Prives C (2010) P53: The Most Frequently Altered Gene in Human Cancers. Nature Education 3(9): 6.
- Callaway E (2015) How Elephants Avoid Cancer. Nature News 18534.
- Pak VN (2021) Alpha-fetoprotein and Its Receptor in Fixing the Cancer Brakes. Cambridge Scholars Publishing, England, Tyne, pp. 209.
- Li Y, He H, Jihu R, Zhou J, Zeng R, et al. (2021) Novel Characterization of Myeloid-Derived Suppressor Cells in Tumor Microenvironment. Front Cell Dev Biol 9: 698532.
- Grover A, Sanseviero E, Timosenko E, Gabrilovich DI (2021) Myeloid-Derived Suppressor Cells: A Propitious Road to Clinic. Cancer Discov 11(11): 2693-2706.
- Ostrand-Rosenberg S, Fenselau C (2018) Myeloid-Derived Suppressor Cells Immune-Suppressive Cells That Impair Antitumor Immunity and Are Sculpted by Their Environment. J Immunol 200(2): 422-431.
- Govallo VI (1993) Immunology of Pregnancy and Cancer. Nova Science Publishers, Commack, New York, USA, pp. 310.
- Budhwar S, Verma R, Verma P, Bala R, Rai S, et al. (2021) Estradiol correlates with the accumulation of Monocytic Myeloid-Derived Suppressor Cells in Pre-term birth: A possible explanation of immune suppression in pre-term babies. J Reprod Immunol 147: 103350.
- Zamorina SA, Troynich YN, Loginova NP, Charushina YA, Shardina KY, et al. (2021) Pregnancy-Associated Proteins as a Tool in the Therapy of Autoimmune Diseases and Alloimmune Disorders. In: Rocha A, Isaeva E (Eds.) Science and Global Challenges of the 21st Century - Science and Technology. Perm Forum 2021 Springer, Cham 342: 385-393.
- Jørgensen N, Persson G, Hviid TVF (2019) The Tolerogenic Function of Regulatory T Cells in Pregnancy and Cancer. Front Immunol 10: 911.
- Jewett A, Tseng H, Arasteh A, Saadat S, Christensen RE, et al. (2012) Natural Killer Cells Preferentially Target Cancer Stem Cells; Role of Monocytes in Protection against NK Cell Mediated Lysis of Cancer Stem Cells. Curr Drug Deliv 9(1): 5–16.
- Pak VN (2018) Selective targeting of myeloid-derived suppressor cells in cancer patients through AFP-binding receptors. Future Sci OA 5(1): FSO321.
- Law AMK, Valdes-Mora F, Gallego-Ortega D (2020) Myeloid-Derived Suppressor Cells as a Therapeutic Target for Cancer. Cells 9(3): 561.
- Carnevalli LS, Ghadially H, Barry ST (2021) Therapeutic Approaches Targeting the Natural Killer-Myeloid Cell Axis in the Tumor Microenvironment. Front Immunol 12: 633685.
- Belyaev NN, Abdolla N, Perfilueva Yu, Ostapchuk YO, Krasnoshtanov VK, et al (2017) Daunorubicin conjugated with alpha-fetoprotein selectively eliminates myeloid-derived suppressor cells MDSCs and inhibits experimental tumor growth. Cancer Immunol Immunother 67(1): 101-111.
- Tumino N, Besi F, Martini S, Di Pace AL, Munari E, et al. (2022) Polymorphonuclear Myeloid-Derived Suppressor Cells Are Abundant in Peripheral Blood of Cancer Patients and Suppress Natural Killer Cell Anti-Tumor Activity. Front Immunol 12: 803014.
- Lakhi N, Moretti M (Eds.) (2016) Alpha-Fetoprotein: Functions and Clinical Application. Protein Biochemistry, Synthesis, Structure and Cellular Functions. Nova Science Publisher’s, Inc. Hauppauge, New York, USA, pp. 420.
- Mizejewski GJ (2019) Protein Binding and Interactions with Alpha-Fetoprotein (AFP): A Review of Multiple AFP Cell Surface Receptors, Intracytoplasmic Binding, and Inter Molecular Complexing Proteins. J Mol Cell Biol Forecast 2(1): 1016.
- Suzuki Y, Zeng CQ, Alpert E (1992) Isolation and Partial Characterization of a Specific Alpha-Fetoprotein Receptor on Human Monocytes. J Clin Inv 90(4): 1530–1536.
- Esteban C, Trojan J, Macho A, Mishal Z, Lafarge-Frayssinet C, et al. (1993) Activation of an Alpha-Fetoprotein/Receptor Pathway in Human Normal and Malignant Peripheral Blood Mononuclear Cells. Leukemia 7(11): 1807–1816.
- Smrkolj T, Gubina B, Bizjak J, Kumer K, Fabjan T, et al. (2017) Tumor marker α-fetoprotein receptor does not discriminate between benign prostatic disease and prostate cancer. Adv Clin Exp Med 26(7):1085-1090.
- Sedky HA, Youssef SR, Gamal DA, Houssein HF, Elsalakawy WA (2020) First report of the unique expression of RECAF (receptor for alfa feto-protein) in adult B-NHL/CLL patients. Blood Res 55(4): 253-261.
- Lin B, Wang Q, Liu K, Dong X, Zhu Mand Li M (2021) Alpha-Fetoprotein Binding Mucin and Scavenger Receptors: An Available Bio-Target for Treating Cancer. Front Oncol 11: 625936.
- Pak V, Molchanov O, Vincent M (2007) Treatment of Metastatic Colorectal Cancer with Aimpila a Glycoside/AlphaFetoprotein Complex. J Clin Oncol 25: 3589-3589.
- Sherman I, Boohaker R, Stinson K, Griffin P, Hill W (2021) AFP-Maytansine Conjugate - a Novel Targeted Cancer Immunotherapy. European Society of Medical Oncology (ESMO) Annual Meeting September 16-21, Abstract 2838, poster, pp. 523.






























