Unexpected Chemotherapy Toxicity in a 28-year-Old -Male with localized Colon Cancer
Dr. Zuhair Alzibair*
Department of Medical Oncology, King Abdullah Medical City, Saudi Arabia
Submission: January 13, 2022; Published: January 21, 2022
*Corresponding Address: Zuhair Alzibair, Department of Medical Oncology, King Abdullah Medical City, Saudi Arabia
How to cite this article: Dr. Zuhair A. Unexpected Chemotherapy Toxicity in a 28-year-Old -Male with localized Colon Cancer. Canc Therapy & Oncol Int J. 2022; 20(5): 556046. DOI:10.19080/CTOIJ.2022.20.556046
Introduction
Hereditary non polyposis coli (HNPCC) or Lynch syndrome accounts for 1-3% of all colorectal cancer diagnosis. It is characterized by increased lifetime risk of colorectal cancer (30%- 73%) [1-3]. Lynch syndrome is caused by a defect in mismatch repair MMR system that can lead to carcinogenesis. The mammalian DNA mismatch repair (MMR) system consists of several proteins that play important roles in repair of base pair mismatch mutations and in maintenance of genomic integrity [4]. Microsatellite instability account to ≈ 12 % of colorectal cancer cases and associated with low recurrence rate after surgery [5]. Examples for mismatch repair genes are MLH1, MLH2, MHS-2, MSH3, MSH5 and PMS2. To date, more than 300 different predisposing mutations are known, mainly affecting the MMR genes MLH1 (∼50%), MSH2 (∼40%) and MSH6 (∼10%.) [6]. Updated international guidelines include MSI status in the decision of adjuvant systemic treatment in stage II colon cancer. MSI-high (MSI-H) has been detected in 4.3% of patients with metastatic colorectal cancer [7]. Although immunotherapy has shown great benefit in stage IV high MSI in comparison to chemotherapy, its benefit role in stage III colon cancer is not well established.
The case
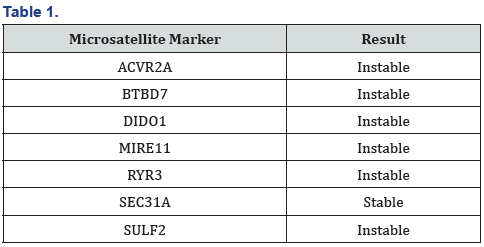
We are reporting a case of 28 years old patient male with stage III Colon cancer(T3N1M0), who developed significant hepatic and bone marrow toxicity and after 3 three months of adjuvant CAPOX). He presented initially with symptoms and signs of acute bowel obstruction. Urgent CT scan showed obstructive caecal mass with no evidence of distant metastasis. He underwent right hemicolectomy in March 2020. Histopathology revealed mucinous adenocarcinoma (T3) with three positive lymph nodes out of twenty-six (N1). MSI was requested upon histopathology review in our institution: - microsatellite unstable adenocarcinoma (6/7 markers were unstable), which could indicate the presence of Lynch syndrome (Table 1).
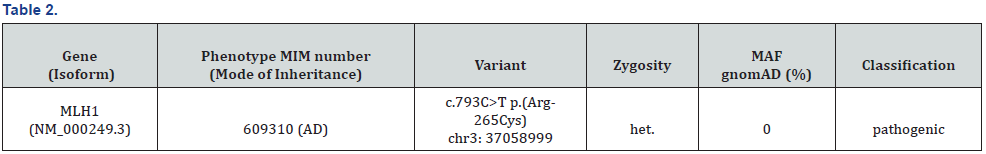
The post-operative course was unremarkable. The decision was to start adjuvant chemotherapy for three months (according to the results of IDEA trial) after explaining the rationale and side effects of treatment. Given the MSI status and his strong family history (mother died because of colon cancer at the age of 40), he was referred to the oncology genetic clinic. Diphenhydramine dehydrogenase enzyme (DPD) activity test was not performed before chemotherapy as it was not a routine test in the institution at that time. He tolerated the first cycle of CAPOX well. However, he developed persistent grade II nausea and fatigue after the second cycle. He was in need for dose reduction with the subsequent two cycles. It was ten days after the last cycle when the patient presented with nausea, abdominal pain grade II, and jaundice (liver function test was normal at time of chemotherapy assessment. His new total bilirubin was 3.6mg/dl CTC AE 4.03 grade III), direct bilirubin was 1.97 mg/dl, AST 145 U/L (CTC AE 4.03 grade I) and ALT was 605 (CTC AE 4.03 U/L grade II). There was evidence of bone morrow depression: platelet count was 40,000 CTC AE 4.03 grade III) (baseline 143.000). HB 8.8 gm/ dl (baseline 11.5) CTC AE 4.03 grade II) but no neutropenia. Rest of chemotherapy (capecitabine) was stopped, and he was kept on supportive. His liver function test started to improve after one week. His bilirubin normalized in two weeks approximately and his hemoglobin and platelet count in around four weeks. He was on close and regular follow up: after one year he asymptomatic, no signs of local or distant recurrence his liver enzymes are still two folds high. Repeated CT scans and colonoscopy showed no evidence of local recurrence or distant metastasis. The patient was assessed by the oncogenetist, Hereditary Colon Cancer Multi-Gene Panel was requested: he was having germline MLH1 mutation (Table 2).
Discussion
Prediction of adjuvant chemotherapy toxicity in primary colon cancer depends on several factors, including age, performance status and patient’s co-morbidities specially those are associated with increasing chances of having neurotoxicity e.g. Diabetes mellitus and other causes of peripheral neuropathy. Whether the presence of microsatellite instability and other genomic profile testing might impact the benefit [5] or the toxicity of chemotherapy in stage III colon cancer is unclear at present.
Adding oxaliplatin in adjuvant chemotherapy may overcome negative impact of 5-FU on colon cancers with MSI-H/MMR-D. Fluorouracil-based adjuvant chemotherapy benefited patients with stage II or stage III colon cancer with microsatellite-stable tumours or tumours exhibiting low-frequency microsatellite instability but not those with tumours exhibiting high-frequency microsatellite instability [5,8]. A retrospective study compared FOLFOX 4 modified to FOLFOX6 in metastatic colon cancer, showed that patients with MSI-H colon cancer are more sensitive to a higher dose of FOLFOX, although there was no difference in overall survival [9].
Various studies had not specified the toxicity profile or patient reported outcomes in patients with MSI-H colon cancer who are on adjuvant chemotherapy. This case report might highlight the importance of doing further subset analysis to evaluate the impact of chemotherapy side effects in such patients. In the KEYNOTE 177 study, evaluating immunotherapy in h MSI-high advanced colorectal cancer, treatment-related adverse events of grade 3 or higher occurred in 22% of the patients in the pembrolizumab group, as compared with 66% (including one patient who died) in the chemotherapy group. Grade 3 toxicity in AST and ALT were comparable in both arms (3-4%) [10]. In view of the significant response and survival benefit of immunotherapy in MSI-H in metastatic colorectal cancer [10,11], this treatment option can be explored further in localized colon cancer in adjuvant setting.
References
- Lynch HT de la CA (2003) Hereditary Colorectal Cancer. N Engl J Med 348(10): 919-932.
- Win A, Young J, Lindor N, KT-J, Dennis J Ahnen, et al. (2021) of clinical, 2012 undefined. Colorectal and other cancer risks for carriers and noncarriers from families with a DNA mismatch repair gene mutation: a prospective cohort study. J Clin Oncol 30(9): 958-964.
- Engel C, Loeffler M, Steinke V, Nils Rahner, Elke Holinski-Feder, et al. (2012) Risks of less common cancers in proven mutation carriers with lynch syndrome. J Clin Oncol 30(35): 4409-4415.
- Hassen S, Ali AA, Kilaparty SP, Al-Anbaky QA, Majeed W, et al. (2016) Interdependence of DNA mismatch repair proteins MLH1 and MSH2 in apoptosis in human colorectal carcinoma cell lines. Mol Cell Biochem 412(1–2): 297–305.
- Kim ST, Lee J, Park SH, Park JO, Lim HY, et al. (2010) Clinical impact of microsatellite instability in colon cancer following adjuvant FOLFOX therapy. Cancer Chemother Pharmacol 66(4): 659-667.
- Peltomaki P (2001) Deficient DNA mismatch repair: a common etiologic factor for colon cancer. Hum Mol Genet 10(7): 735–740.
- Kim ST, Kim HK, Lee J, Park SH, Lim HY, et al. (2018) The impact of microsatellite instability status and sidedness of the primary tumor on the effect of bevacizumab-containing chemotherapy in patients with metastatic colorectal cancer. J Cancer 9(10): 1791-1796.
- Liu J, Wang B, Fang W (2020) Microsatellite instability and sensitivity to fluoropyrimidine and oxaliplatin containing first-line chemotherapy in metastatic colorectal cancer. Eur J Hosp Pharm 27(5): 267-270.
- Des Guetz G, Mariani P, Cucherousset J, Benamoun M, Lagorce C, et al. (2007) Microsatellite instability and sensitivitiy to FOLFOX treatment in metastatic colorectal cancer. Anticancer Res 27(4C): 2715-2719.
- Andre T, Shiu K-K, Kim TW, Jensen BV, Jensen LH, et al. (2020) Pembrolizumab in Microsatellite-Instability–High Advanced Colorectal Cancer. N Engl J Med 383(23): 2207–2218.
- Keating M, Giscombe L, Tannous T, Hartshorn K (2019) Prolonged Treatment Response to Pembrolizumab in a Patient with Pretreated Metastatic Colon Cancer and Lynch Syndrome. Mayordomo JI, editor. Case Rep Oncol Med 2019: 3847672.






























