The Waxy Agglomeration-Ceruminous Adenoma
Anubha Bajaj*
Histopathologist in A B Diagnostics, New Delhi, India
Submission: December 14, 2021; Published: January 10, 2022
*Corresponding Address: Anubha Bajaj, Histopathologist in A B Diagnostics, New Delhi, India
How to cite this article: Anubha B. The Waxy Agglomeration-Ceruminous Adenoma. Canc Therapy & Oncol Int J. 2022; 20(4): 556041. DOI:10.19080/CTOIJ.2022.20.556041
Preface
Ceruminous glands are specialized, apocrine glands discerned within cartilaginous portion of external auditory canal. Preponderant function of the glands is to secrete cerumen. Besides, primary neoplasms of the external auditory canal are extremely exceptional. Ceruminous adenoma is an exceptional, benign neoplasm arising from cerumen secreting, modified apocrine, ceruminous glands. However, ceruminoma as a terminology is contemplated to be archaic. Notwithstanding, ceruminous gland neoplasia can be considered as potentially malignant or of “uncertain malignant potential” as biological behaviour of the tumefaction remains undefined. The neoplasm can be challenging to discern on account of a variety of emergent clinical representations within the external auditory canal. Cogent tumour categorization is possible on histopathological examination. The neoplasm is associated with favourable prognosis following comprehensive surgical excision.
Disease Characteristics
The neoplasm occurs within external auditory canal, especially incriminating the extraneous segment which demonstrates a preponderance of ceruminous glands. Nevertheless, areas such as parotid gland or middle ear may be implicated [1]. The exceptional, benign ceruminous adenoma usually appears beyond > 50 years although adolescents may exhibit the neoplasm. Ceruminous adenoma commonly emerges in adults between 24 years to 85 years. An equivalent gender predilection is delineated [2,3]. Malignant metamorphosis occurs in an estimated 70% instances of ceruminous adenoma with the emergence of possible intracranial extension and distant metastasis. Malignant metamorphosis is accompanied by pain and facial nerve paresis [2,3]. Concurrent conditions such as chronic otitis media, infection of parotid gland and cervical lymphadenopathy appear as coincidental features [2,3].
Clinical Elucidation
The gradually progressive neoplasm represents with otalgia, yellowish ear discharge, pruritus, chronic irritation, impediment of external auditory canal and conductive deafness. Intermittent pyrexia or haemorrhage may occur. Tinnitus, vertigo or cranial nerve palsies are usually absent. Tumefaction can be associated with ruptured tympanic membrane or otorrhea [2,3]. Predominant clinical manifestations are hearing loss, pyrexia, persistent earache, minimal or moderate otalgia and otorrhoea with concomitant chronic otitis media. Bilateral cervical lymphadenopathy may accompany the neoplasm. Concurrence with preceding trauma or surgical intervention is usually absent [3,4]. Comorbid conditions such as atrial fibrillation, diabetes mellitus, arterial hypertension or glaucoma can be discerned [3,4].
Histological Elucidation
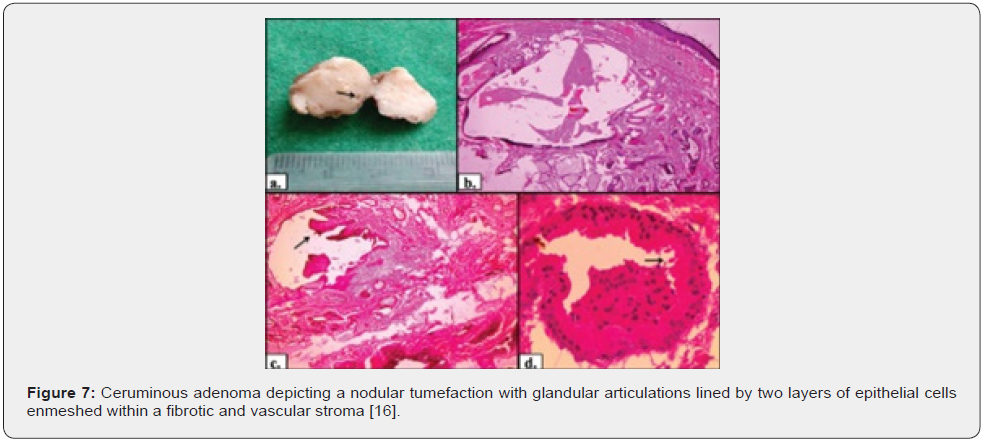
Upon gross examination, ceruminous adenoma emerges as a reddish, polypoid, solid, well circumscribed tumefaction with a smooth or ulcerated surface or a partially skin covered, fusiform nodule of magnitude varying from 0.4 centimetres to 2.0 centimetres or as a smooth, spherical, un-encapsulated tumefaction discharging serosanguinous or yellowish fluid upon pressure. Cystic alterations are infrequently discerned. Cut surface depicts a subcutaneous, well circumscribed, solid or cystic lesion [3,4].
Upon cytology, the grey/white, cellular aspirate demonstrated cohesive, mono-layered sheets and papillary fragments of epithelial cells. Tumour cells are imbued with abundant, eosinophilic cytoplasm with spherical to elliptical, bland nuclei and inconspicuous nucleoli. Spindle-shaped, myoepithelial-like cells and few plasmacytoid cells are also observed. Eosinophilic stromal fragments are preponderantly composed of fibroblastic, spindle-shaped cells. Cellular stromal fragments appear commingled with epithelial cell aggregates [3,4]. The neoplasm can uniquely manifest papillae, dense, cohesive cellular clusters of epithelial and myoepithelial cells and disseminated plasmacytoid cells. The cellular elements are admixed within fibromyxoid stromal fragments [3,4].
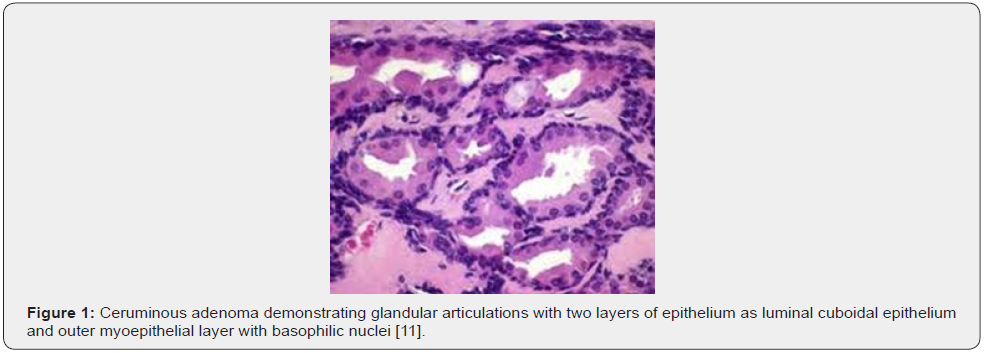
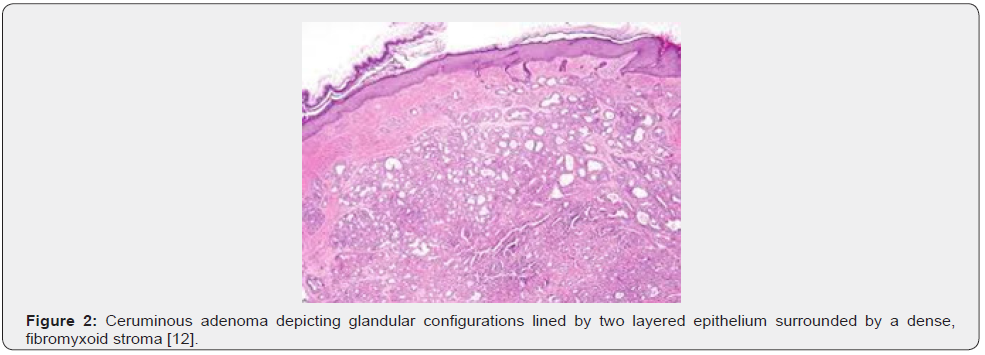
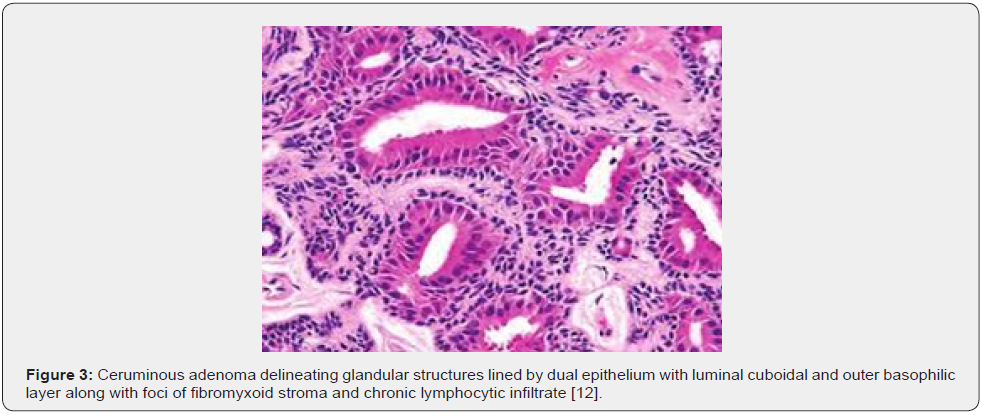
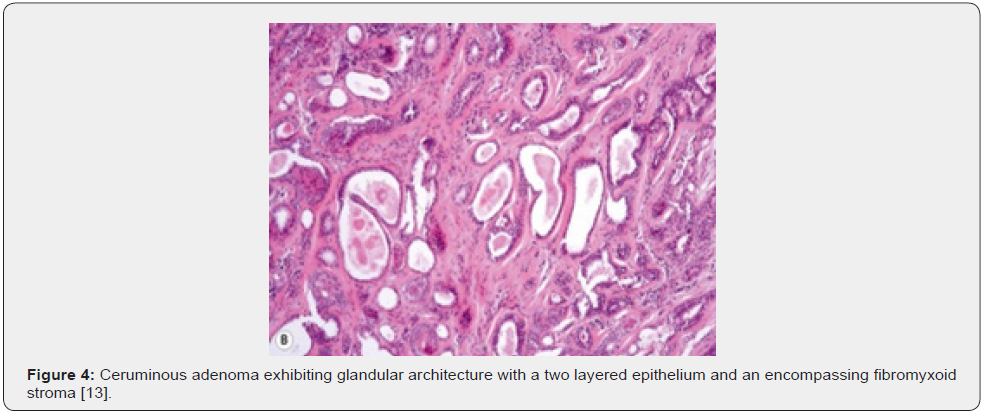
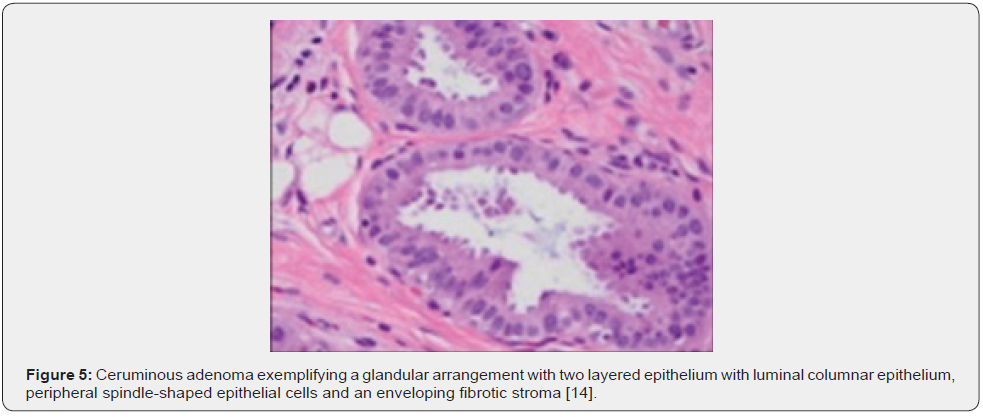
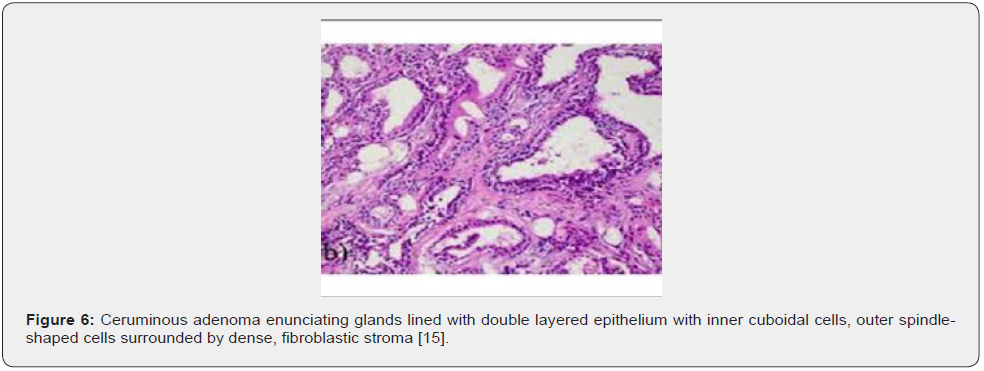
Microscopic variations of architectural pattern of the un-encapsulated neoplasm are delineated as densely accumulated glands, foci of cystic dilatation, solid areas, and configuration of papillary structures [3,4]. Upon microscopy, a cystic lesion layered with epithelium is denominated. The lining epithelium is comprised of dual cell population wherein the intrinsic, luminal epithelial layer is composed of tubules of cylindrical, cuboidal or low columnar epithelial cells of intermediate magnitude imbued with intensely eosinophilic cytoplasm, spherical nuclei, and focal decapitation secretion. Occasionally, cylindrical cells demonstrate cytoplasmic projections extending into tubular lumina. Extraneous cell layer configures fascicles, miniature solid cell aggregates or exceptional tubules articulated of elliptical to spindle- shaped myoepithelial cells with scanty cytoplasm and elongated, hyperchromatic nuclei. Cellular and nuclear atypia or mitotic activity is absent [4,5].
Tumour is composed of proliferating cells configuring tubular glands, solid sheets, papillae and cysts. Tubules and glands are coated with a dual cell population. Luminal epithelial cells appear as tall, columnar to cylindrical cells imbued with abundant, granular, eosinophilic cytoplasm and bland, spherical to elliptical nuclei. Luminal epithelial cells demonstrate focal decapitation secretion whereas peripheral epithelial cell layer is basaloid. Tumour cell articulations are circumscribed by a fibromyxoid stroma [4,5]. The un-encapsulated, well circumscribed neoplasm demonstrates glandular proliferation with distinctive cribriform, solid, cystic or papillary configuration. Tumour glands are layered with an inner layer of cuboidal to columnar cells incorporated with eosinophilic cytoplasm and apical snouts constituted of decapitation secretion. The peripheral myoepithelial layer is composed of spindle-shaped cells with hyperchromatic nuclei. Intrinsic luminal epithelial cells demonstrate yellow-brown, granular, cerumen pigment. The circumscribing stroma is hyalinised. Cellular or nuclear pleomorphism, mitotic activity, tumour infiltration or tumour necrosis is absent [4,5].
Tumefaction is embedded within a fibrous, focally hyalinised stroma. An attenuated fibrous capsule may partially circumscribe the neoplasm and appears non-contiguous with superimposed stratified squamous epidermal layer [4,5]. Upon immunohistochemistry, dual cell population can be confirmed. Luminal epithelial cells are immune reactive to cytokeratin 7 along with membranous reactivity to CD117 and p53. Peripheral or basal epithelial cells are immune reactive to cytokeratin 5/6, S100 protein and p63. Cerumen can be stained with periodic acid Schiff’s (PAS) stain or mucicarmine [5,6]. Ki-67 proliferation index appears at around 60% in luminal nuclei and nearly 5% in basal nuclei. Apocrine gland-related antigen or gross cystic disease fluid protein 15 (GCDFP-15) is focally expressed by the neoplastic cells. Immunohistochemistry is identical to immune staining pattern discovered in normal ceruminous glands [5,6] (Figures 1-7).
Differential Diagnosis
Ceruminous adenoma requires a segregation from •ceruminous adenocarcinoma which is an infiltrative neoplasm configured of irregular glands and frequently accompanied by perineural invasion. Tumour cell nuclei are imbued with prominent nucleoli. Ceruminous granules are usually absent. Cellular and nuclear pleomorphism, foci of tumour necrosis, enhanced mitotic activity and atypical mitotic figures are delineated [6,7].
neuroendocrine adenoma of the middle ear demonstrates variable neoplastic configurations such as cellular sheets, solid areas, trabeculae, cystic articulations, cribriform pattern, or glandular structures. Generally, papillary architecture is absent. Tumour glands or tubules are layered with uniform, singular layer of cuboidal or columnar epithelial cells incorporated with variable quantities of eosinophilic cytoplasm and spherical to elliptical, hyperchromatic nuclei with eccentric nucleoli. Neoplastic cells may appear plasmacytoid and demonstrate significant cellular and nuclear pleomorphism. The circumscribing stroma is sparse, fibrotic or myxoid. Mitotic activity is minimal to absent. Necrosis is absent [6,7].
Paraganglioma demonstrates a classic, organoid pattern with the configuration of a “zellballen” or nesting of tumour cells. Tumefaction is constituted of centric, spherical, or elliptical chief cells imbued with abundant, eosinophilic, granular, or vacuolated cytoplasm and uniform nuclei with dispersed chromatin. Spindleshaped, basophilic, peripheral sustentacular cells circumscribe tumour cell nests and are immune reactive to S100 protein. Tumour cells may depict cellular and nuclear pleomorphism. Tumour cell aggregates are subdivided by prominent fibrovascular stroma. Occasionally, dense fibrous stroma may encircle clusters of tumour cells. An infiltrative pattern of tumour evolution is encountered [6,7].
Additionally, various neoplasms arising within external auditory canal such as exostosis, osteoma, eosinophilic granuloma, cholesteatoma, cartilaginous choristoma, extraadrenal paraganglioma, congenital cysts of branchial arch origin, pleomorphic adenoma or associated tumours of the parotid gland and meningioma require a segregation. Precise distinction is possible upon competent histological evaluation and pertinent immunohistochemistry [6,7].
Investigative Assay
Upon physical examination, a spherical, reddish, tender, soft tissue mass can be observed within the cartilaginous segment of external auditory canal associated with mucopurulent discharge. The neoplasm may exude a yellowish fluid upon pressure. Ear discharge can be subjected to culture and sensitivity for appropriate bacteriological evaluation [8,9]. Audiometry delineates conductive deafness of the incriminated ear. Cogent cytological assessment with fine needle aspiration circumvents irrelevant, extensive surgical intervention and ameliorates prognostic outcomes [8,9]. Computerized tomography (CT) demonstrates a cystic lesion arising within the external auditory canal. Bone lysis of adjoining osseous fragments is absent. Middle ear may depict accumulation of fluid [8,9].
Therapeutic Options
Intravenous antibiotics can be administered. Oral antibiotics or anti-inflammatory medications are usually inefficacious. Neoplasm confined to external auditory canal can be subjected to comprehensive surgical excision with a trans-meatal approach [8,9]. Complete surgical extermination with a broad, tumourfree perimeter is recommended, thereby ensuring an extended tumour-free survival. Postoperative tumour alleviation is accompanied by reduction of ear discharge, disappearance of otalgia and amelioration of hearing [9,10]. Ruptured tympanic membrane may heal [10]. Ceruminous adenoma is usually devoid of tumour reoccurrence as estimated by a mean follow-up of 15 years. Nevertheless, tumour reoccurrence may ensue with incomplete surgical excision or appearance of residual tumour following surgical resection [9,10].
References
- Batsakis JG, Hardy GC, R H Hishiyama (1967) Ceruminous gland tumours. Arch Otolaryngol 86(1): 66–69.
- Psillas GP, Krommydas A, Georgia Karayannopoulou, Kyriakos Chatzopoulos, Jean Kanitakis et al. (2015) Ceruminous adenoma of the external auditory canal: A case report with imaging and pathologic findings. Case Rep Med 2015: 359627.
- Das R, Nath G, Sangita Bohara, Shivanjali Raghuvanshi (2017) Ceruminous adenoma: a rare tumour diagnosed on cytology with histological correlation. J Cytol 34(3): 168-170.
- Varshney H, Taneja V, MK Taneja (2014) Ceruminous gland adenoma. Indian J Otol 20(1): 41-43.
- Mahanta AA, Andola SK (2015) FNAC diagnosis of ceruminous adenoma of the external auditory canal with histological correlation. PJSR 8(1): 60-62.
- Thompson DR, Nelson BL, E Leon Barnes (2004) Ceruminous adenomas: a clinicopathologic study of 41 cases with a review of the literature. Am J Surg Pathol 28(3): 308-318.
- Giuseppe M, Serena B, Sandro Bosco, Cristiano Di Nota, Vincenzo Savastano et al. (2011) Adenoma of the ceruminous gland (Ceruminoma). Otology and Neurotology 32(2): e14–e15.
- A Selcuk , S Ensari, M A Cetin, S Dizbay Sak, H Dere (2007) Ceruminous gland carcinoma of the external auditory canal presenting as chronic otitis media. B-ENT 3(4): 195–199.
- Elsurer C, Senkal HA, Dilek Ertoy Baydar, Levent Sennaroglu (2007) Ceruminous adenoma mimicking furunculosis in the external auditory canal. Eur Arch Otorhinolaryngol 264(3): 223–225.
- Lassaletta L, Patron M, Javier Oloriz, Rosa Perez, Javier Gavilan (2003) Avoiding misdiagnosis in ceruminous gland tumours. Auris Nasus Larynx 30(3): 287–290.
- Image 1 Courtesy: Wikipedia
- Image 2 and 3 Courtesy: Dr Lester DR Thompson M.D
- Image 4 Courtesy: Basic medical key
- Image 5 Courtesy: Indian Journal of Cytology
- Image 6 Courtesy: Research gate
- Image 7 Courtesy: Journal of Cytology






























