Liver Metastasis as the First Manifestation of Occult Breast Carcinoma
Welington Lombardi*, Luciana Borges Lombardi, Luis Henrique de Carvalho, Carolina Barcha Santos, Ana Carolina Abud Ferreira, Beatriz Berchielli Moreno, Maiara Brandão Sampaio de Mendonça and Flavia Vicentin Silva
University of Araraquara, Uniara 14801-308, Brazil
Submission: December 26, 2021; Published: January 07, 2022
*Corresponding Address: Welington Lombardi, University of Araraquara, Uniara 14801-308, Rua das Magnolias, 150, Zip Code: 14802-795, Araraquara-SP, Brazil
How to cite this article: Welington L, Luciana Borges L, Luis Henrique d C, Carolina Barcha S, Ana C A F, et al. Liver Metastasis as the First Manifestation of Occult Breast Carcinoma. Canc Therapy & Oncol Int J. 2022; 20(3): 556040. DOI:10.19080/CTOIJ.2022.20.556040
Abstract
Occult breast cancer is a rare entity, accounting for less than 1% of cases. When present, it usually presents as metastases in axillary lymph nodes and absence of tumor in the breast tissue and, therefore, exclusively hepatic metastasis is uncommon. This paper aims to present the case of a 61-year-old patient with metastasis in the left hepatic lobe with histology compatible with breast carcinoma and absence of malignant findings in the breast or in axillary lymph nodes. This is an observational and descriptive study based on data from medical records, with a theoretical framework and review using google scholar, pubmed and scielo platforms. The work was approved by the research ethics committee under number caae 47633321.0.0000.5383. Occult breast cancer usually presents with metastatic axillary disease, and cases of primary breast origin diagnosed exclusively with metastatic liver lesion are very rare. The use of imaging tests such as mammography, ultrasound, magnetic resonance, tomography and pet-ct, as well as immunohistochemistry revealing the presence of gata-3 and estrogen receptors, among other proteins are essential for diagnostic confirmation.
Keywords: Breast cancer; Occult breast cancer; Liver metastasis
Introduction
Breast cancer is a heterogeneous disease both for its metastatic capacity and for its rapid spread, being the most common malignant neoplasm in women worldwide and, therefore, it is a subject of priority in research and medical studies [1]. It is estimated that, in the world, one in eight women will develop breast cancer, with approximately 1 million and 700 thousand new cases each year, showing that progress in the field of prevention remains slow, globally [2]. Furthermore, incidence and mortality rates are likely to increase significantly over the next 10 years, with disproportionately high growth expected in developing countries. Thus, countries like Brazil have this trend towards an increase in the incidence and mortality of breast cancer [2,3]. Within the cases of malignant breast neoplasms, an expressive percentage of 90 to 95% are due to environmental influences and factors related to the patient’s lifestyle, while only 5 to 10% of the cases are originated from genetic diseases [2]. Its recurrence and increased morbidity are increased by the coexistence of these two factors. Environmental and lifestyle factors include: ionizing radiation, nulliparity, late pregnancy, short breastfeeding periods, early menarche and late menopause, hormone therapy, alcohol consumption, smoking, obesity and sedentary lifestyle. In addition to the aforementioned risk factors, low socioeconomic status is also associated with an increased risk of aggressive premenopausal breast cancers, late diagnosis and worse survival [4,5].
The prognosis for breast cancer tends to be favorable when detection is early and treatment is effective. Early detection includes screening mammography and breast self-examination, as well as not being exposed to risk factors, maintaining a healthy diet and practicing physical activity would be protective factors against breast cancer [2,4]. However, 20 to 30% of the cases will develop metastases, having as the main sites the bones, lungs, liver and brain, all of them associated with low survival, with an average of only 24 months1. However, the liver is a site of high incidence of breast cancer recurrence reaching, in some studies, rates of 50% of metastases [4,6-8].
In the United States, more than half of breast carcinomas are detected by screening mammography and approximately one third of diagnoses present as a palpable breast mass [9]. Other less common forms of presentation are: palpable axillary mass, nipple discharge, nipple inversion, asymmetry breast, erythema of the skin and thickening of the breast skin with an orange peel appearance. In addition, in the event of diagnosis, 31% of breast carcinomas have already reached the regional lymph nodes, but only 6% present as metastatic cases [9].
Occult breast carcinoma is extremely rare, and in these cases it is common to observe axillary metastases in the absence of a primary breast tumor on physical examination, imaging tests or preoperative biopsies [10]. Therefore, there is no detectable lesion in the breast, but the histology and/or immunohistochemistry of the metastatic lesion confirms the mammary origin of the tumor. However, it is noteworthy that most reports on occult breast cancer addressed cases whose MRI was not yet considered standard for preoperative evaluation, therefore, it is estimated that there are more cases with misdiagnosis in the preoperative evaluation [10,11].
It is important to highlight that the diagnosis of occult breast cancer is related to breast reduction surgery. In these surgeries, when the removed tissue was sent for histopathological analysis, it was observed that the incidence was 0 to 2% in women without a personal history of breast cancer and 0 to 5.5% in women with breast cancer anterior in the contralateral breast [12]. Furthermore, regarding the incidence of occult breast cancer that manifested with metastasis in axillary lymph nodes, the value found was approximately 0.3% to 1%. Therefore, in the case of occult breast cancer with suspected liver metastasis, non-invasive investigations may not provide a diagnosis, so that liver biopsy is the definitive procedure of choice [13]. Then, the morphological and immunophenotypic changes found, if they indicate occult breast carcinoma, could guide towards a definitive diagnosis and result in a faster initiation of a possible more effective therapy, since early diagnosis has therapeutic and prognostic implications [13,14].
We report the case of a patient with occult breast cancer, with no detectable breast or axillary lymph node lesions, discovered after a single finding of metastasis in the left hepatic lobe. Descriptive and observational study that used the Google Scholar, Pubmed and Scielo research platforms as a theoretical framework. The study was approved by the Ethics and Research Committee under number CAAE 47633321.0.0000.5383 of November 16, 2021. In the literature studied, only one case of occult breast carcinoma that presented as gastrointestinal metastasis without involvement of the axillary lymph nodes was found which reinforces the importance of this report [13].
Case Report
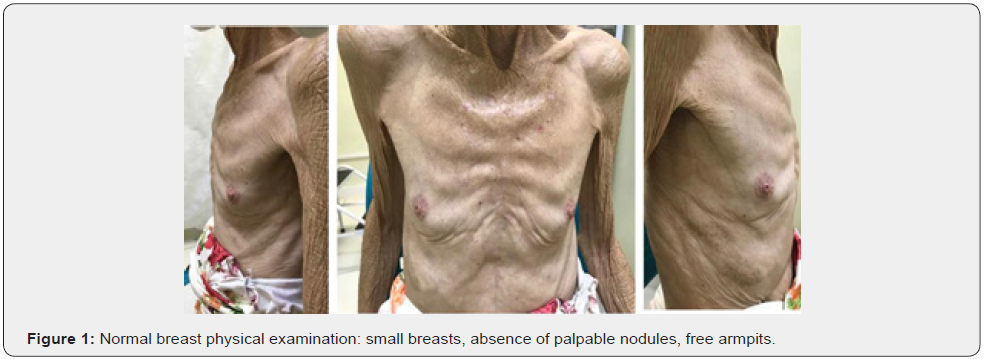
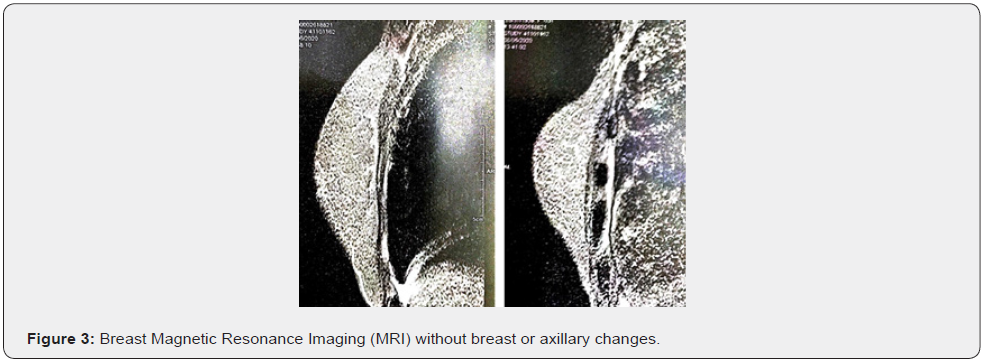
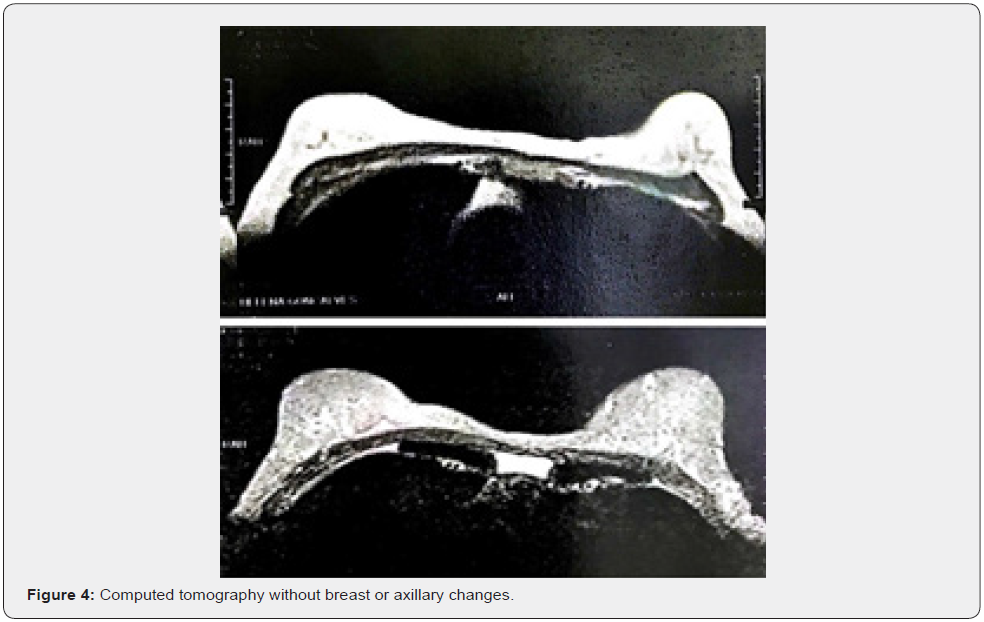
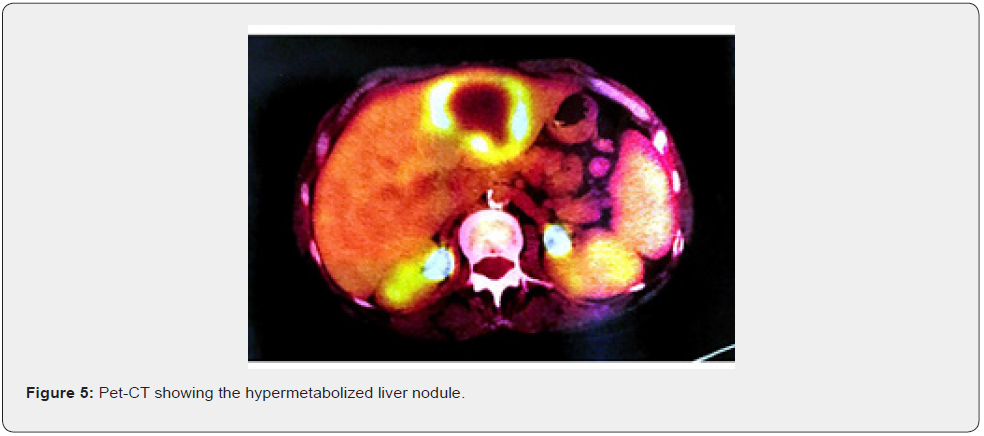
Female patient, 61 years old, born in the city of Sao Paulo, Brazil, smoker, coronary artery disease and with a pathological history of cervical cancer EC IB2, treated in 2017. The patient complained of weight loss, abdominal and pelvic pain and hematuria. Physical examination revealed the presence of a hardened nodule in the epigastric region, painful on abdominal palpation. Total abdominal ultrasound showed a segment II liver lesion. Then, abdominal computed tomography confirmed a segment II lesion in the liver, measuring 5.8 x 4.0 cm, with dilatation of the biliary tract distally to the lesion and a noncapturing oval image in the head of the pancreas. The initial suspicion was liver metastasis from uterine cervix carcinoma, but core needle biopsy and immunohistochemical study of the sample confirmed the presence of malignant epithelioid neoplasia with breast characteristics in fibrous connective fragments. After these findings, all examinations to investigate breast pathology were performed. The physical examination of the breast did not show any changes (Figure 1), the mammogram was normal (BI-RADS 2) (Figure 2), the breast ultrasound did not show any abnormality (BI-RADS 2), the MRI of the breasts was also normal and there was no evidence of any breast or axillary lesion (BI-RADS 2) (Figure 3). Chest CT was normal (Figure 4). The Pet-CT examination also did not reveal breast or axillary changes, revealing only the liver lesion with hypermetabolism (Figure 5). However, abdominal CT showed liver damage which was confirmed by Pet-Scan. Then, partial hepatectomy and cholecystectomy were indicated as surgical treatment.

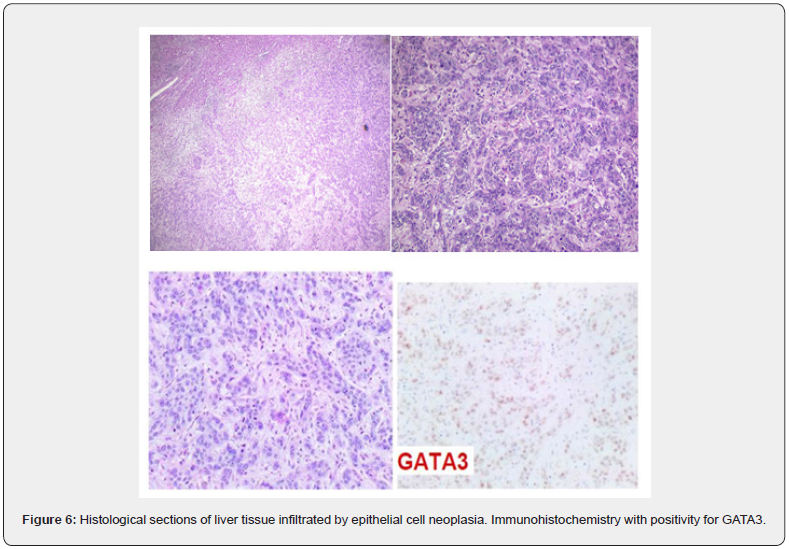
The anatomopathological examination of the surgical specimen showed an irregular nodule measuring 6.5 x 6.0 x 2.3 cm, with intense tumor necrosis, mitotic index of 8 mitoses/10 CGA, without perineural invasion, confirming that it was a malignant epithelioid neoplasm with breast characteristics. Immunohistochemistry revealed positive estrogen receptor (ER) in 50% of neoplastic cells, positivity of antibody P53 in 40%, positivity of antibody KI-67 in 40% of neoplastic cells, positivity for cytokeratins 1, 5, 10 and 14 and positivity for GATA-3. The proteins CDX2, ANXA10, TTF-1 and villin were negative, as well as the markers CA19-9, CEA 17-7 and AFP2 73, corroborating the primary origin of the breast. In the clinical follow-up, the patient has developed bone metastases, diagnosed by bone scintigraphy and died after 04 months of follow-up (Figure 6).
Results and Discussion
Occult breast cancer is a rare form of breast cancer and accounts for less than 1% of cases10. These are cases that progress with metastases to axillary lymph nodes in the absence of any clinical, radiological or pathological finding of a primary breast tumor. Due to the rarity of cases and limited surgical experience, cases of occult breast cancer present several challenges in diagnosis and treatment. In this context, the case reported presented liver metastasis without identifiable breast lesion and without involvement of axillary lymph nodes. The investigation of the breasts by complementary exams such as mammography, breast US, chest CT, breast MRI and Pet-CT did not show signs of breast injury, so that liver injury was only correlated through the immunohistochemical study. According to the literature, in cases of occult breast cancer, mammography, breast US and CT are commonly used to identify the lesion, which is in agreement with the case reported [10,15].
As for liver metastases, they can compromise the organ’s function and, if not promptly treated, are associated with a poor prognosis, which can lead to death in 4 to 8 months, as average. The main treatment indicated for these cases would be chemotherapy, however, even with systemic improvement, the average survival of patients with metastatic disease of breast origin is approximately 18 to 24 months and, despite the transient response to chemotherapy or endocrine therapy, most patients present progressive changes after 1 to 2 years [6].
Further investigation of the molecular phenotype of distant metastasis is essential for discovering future metastases and for the early initiation of effective treatment. Although the main metastatic pathway in breast cancer is the lymphatic pathway, hematogenous dissemination is also observed, especially in the most aggressive ones. The spread of cancer cells from the primary site to distant organs through the bloodstream is not random, as some types of cancer cells have molecular alterations that preferentially affect specific organs, gathering themselves in them. Thus, the implantation fate involves the escape of cancer cells from the primary tumor through the interaction with the microenvironment to the target organ [16].
In molecular investigation, the expression of the estrogen receptor (ER) has important relationships with breast carcinomas. Patients with ER-positive tumors, as presented by the patient in the case, have a better survival than patients with ERnegative tumors, which occur more frequently in young women. In addition, ER-positive tumors behave as predictive markers of response to hormonal therapies and represent a heterogeneous subgroup, whose treatment will depend on tumor pathological characteristics, such as histological grade, nuclear grade and lymph node status [14]. ER-positive tumors are generally well differentiated while ER-negative tumors are poorly differentiated and do not respond to hormonal therapies [17].
The positivity for the GATA-3 protein, associated with morphological aspects and previous clinical data, favored the breast origin of the metastatic carcinoma for the patient in this case. Studies using gene expression profiles and immunohistochemistry showed that GATA-3 expression in breast cancer is closely associated with ER and tumor subtype differentiation into luminal A and luminal B17. The explanation lies in the fact that the GATA family proteins are transcription factors “in zinc finger”, involved in embryogenesis and development. Therefore, the GATA-3 protein in breast epithelial cells acts to maintain a differentiated state. Furthermore, other studies show that GATA-3 genes can be transfected by breast cancer lines and that many genes induced by GATA-3 are also present in luminal A gene clusters. Another point to be highlighted would be that low levels of GATA- 3 could indicate a subgroup of ER-positive tumors with a higher risk of recurrence [14].
Despite the presence of liver metastasis from primary breast carcinoma, according to the literature, the patient’s liver worsening is rarely caused by the dissemination of the primary tumor, which makes it difficult for a more accurate diagnosis. An important and challenging impasse is primary malignancy, which can be hidden in nonspecific and inconclusive radiological findings, laboratory results, and clinical presentation. Occult breast cancer usually presents with metastases or findings in the axillary lymph nodes, which contrasts with this case report, as liver metastasis occurred without any involvement of the axillary lymph nodes [15].
Liver damage was visualized by abdominal CT and by Pet-CT with local hypermetabolism [18]. As for the presence of intense and extensive hepatic necrosis, this finding can be explained by the fact that tumor cells spread throughout the tissue and compete with hepatocytes for oxygen and nutrients, in short, coursing with hemodynamic instability that can generate a decrease in blood volume arterial and resulting in an even greater hepatic impairment [18]. Furthermore, the diffuse invasion of tumor cells into the liver parenchyma leads to acute liver decompensation. It is noteworthy that the liver parenchyma is not always involved, which explains the fact that its surface remains smooth, smooth, without masses or nodularities despite the high degree of tumor invasion [13,18].
As for treatment, the main therapies to treat liver metastasis from occult breast cancer are: surgical resection, transcatheter arterial chemoembolization (TACE), percutaneous radiofrequency ablation (RFCA), ethanol injection and microwave ablation [10]. For the treatment of occult breast cancer itself, according to the literature, two recommendations are suggested: axillary lymph node dissection with complete breast irradiation or axillary lymph node resection with total mastectomy. Considering the age of the patient reported in the study and the urgent need to perform a hepatectomy, the breast therapy chosen at the time was the expectant approach [8,10].
Thus, although breast cancer is one of the most common malignancies in women, occult breast cancer with metastatic liver disease is not a common finding. However, there is still a deficit in studies and in retrospective data available so as to understand cases of occult breast cancer with liver metastasis, and there is much to be understood about this disease so that an increasingly earlier diagnosis is possible, which will bring therapeutic implications and positive prognoses. Most cases of acute liver failure due to neoplastic liver infiltration have an extremely poor prognosis, with death occurring a few days after clinical observation. On balance, the early initiation of therapy aimed at the primary etiology is likely to be able to prolong patients’ survival, as well as to improve their quality of life providing additional benefits [9].
Conflict of Interest
The authors declare that does not exist any conflict of interest or financial interest within the scope of this case report.
References
- Anastasiadi Z, Lianos G D, Ignatiadou E, Harissis H V, Mitsis M (2017) Breast cancer in young women: an overview. Updates Surg 69(3): 313-317.
- Kolak A, Kaminska M, Sygit K, Budny A, Surdyka D, et al. (2017). Primary and secondary prevention of breast cancer. Ann Agric Environ Med 24(4): 549-553.
- Urban L A B D, Chala L F, Bauab S P, Schaefer M B, Santos R P, et al. (2017) Breast cancer screening: updated recommendations of the Brazilian College of Radiology and Diagnostic Imaging. Brazilian Breast Disease Society and Brazilian Federation of Gynecological and Obstetrical Associations. Radiol Bras 50(4): 244-249.
- Jeronimo A F A, Freitas A G Q, Mathias W (2017) Risk factors of breast cancer and knowledge about the disease: an integrative revision of Latin American studies. Cienc Saude Col 22(1): 135-149.
- Coughlin S S (2019) Social determinants of breast cancer risk, stage, and survival. Breast Cancer Res Treat 177(3): 537–548.
- Bale R, Putzer D, Schullian P (2019) Local Treatment of Breast Cancer Liver Metastasis. Cancers Basel 11(9): 1341.
- Wu Q, Li J, Zhu S, Wu J, Chen C, et al. (2017). Breast cancer subtypes predict the preferential site of distant metastases: a SEER based study. Oncotarget 8(17): 27990-27996.
- Lin H, Liang Y, Dou X, Chen C, Wei X, et al. (2018) Notch3 inhibits epithelial–mesenchymal transition in breast cancer via a novel mechanism, upregulation of GATA-3 expression. Oncogenesis (7): 59.
- Waks AG, Winer EP (2019) Breast Cancer Treatment: a review. JAMA 321(3): 288–300.
- Macedo FI, Eid JJ, Flynn J, Jacobs MJ, Mittal VK (2016) Optimal Surgical Management for Occult Breast Carcinoma: A Meta-analysis. Ann Surg Oncol 23(6): 1838-1844.
- Terada M, Adachi Y, Sawaki M, Masaya Hattori, Akiyo Yoshimura,et al. (2018) Occult breast cancer may originate from ectopic breast tissue present in axillary lymph nodes. Breast Cancer Res Treat 172(1): 1-7.
- Fitzpatrick SE, Lam TC (2020) Occult Breast Carcinoma Is More Common in Women Undergoing Breast Reduction after Contralateral Cancer: a systematic review and meta-analysis. Plast Reconstr Surg (146): 117e-126e.
- Neal L, Sookhan N, Reynolds C (2009) Occult breast carcinoma presenting as gastrointestinal metastases. Case Rep Med 2009: 564756.
- Voduc D, Cheang M, Nielsen T (2008) GATA-3 Expression in Breast Cancer has a Strong Association with Estrogen Receptor but Lacks Independent Prognostic Value. Cancer Epidemiol Biomarkers Prev 17(2): 365-373.
- Soundararajan R, Naswa N, Karunanithi S, Walia R, Kumar R, et al. (2016) Occult breast primary malignancy presenting as isolated axillary lymph node metastasis - early detection of primary site by 18F-FDG PET/CT. Nucl Med Rev Cent East Eur 19(B): 5-7.
- Xiao W, Zheng S, Yang A, Zhang X, Zou Y, et al. (2018) Breast cancer subtypes and the risk of distant metastasis at initial diagnosis: a population-based study. Cancer Manag Res 10: 5329-5338.
- Hoch RV, Thompson DA, Baker RJ, Weigel RJ (1999) GATA‐3 is expressed in association with estrogen receptor in breast cancer. Int J Cancer 84(2): 122-128.
- Goswami R, Babich M, Farah KF (2011) Occult breast malignancy masquerading as acute hepatic failure. Gastroenterol Hepatol (N Y) 7(1): 62-65.






























