Infections in Patients with Hematologic Malignancies Undergoing Allogeneic Hematopoietic Stem Cell Transplantations
Selda Kahraman* and Seckin Cagirgan
Department of Hematology; 1825 Street YeniGirne Boulevard, Turkey
Submission: August 20, 2021; Published: September 01, 2021
*Corresponding Address: Selda Kahraman, Adress: Medicalpark Izmir Hospital, Department of Hematology; 1825 Street YeniGirne Boulevard No:12; Karsiyaka, Izmir, Turkey
How to cite this article: Selda Kahraman and Seckin Cagirgan. Infections in Patients with Hematologic Malignancies Undergoing Allogeneic Hematopoietic Stem Cell Transplantations. Canc Therapy & Oncol Int J. 2021; 19(4): 556019. DOI: 10.19080/CTOIJ.2021.19.556019
Abstract
Objective: To assess infections in patients undergoing allogeneic hematopoietic stem cell transplantation (HSCT) from related or non-related donors in our unit.
Methods: A total of 219 allogeneic HSCT performed in 199 patients at the bone marrow transplantation unit were retrospectively evaluated.
Results: There were 72 female and 127 male patients, with a median age of 44 years (16-77 y) at the time of transplantation. Neutropenic fever was observed in 209 patients (92.7%) during transplantation. Microbiological growth was detected in the blood, urine, and sputum cultures in 64 (29.2%), 29 (13.7%), and 12 (5.5%) patients, respectively. At follow-up, 78 patients (35.6%) had CMV antigen positivity, and 24(12%) had galactomannan positivity. A comparison of patients with or without microbiological growth in cultures showed no significant differences in terms of age, gender, acute and chronic GVHD, and disease status at transplantation. Patients with positive microbiologic growth in cultures had shorter total and progression-free survival (p < 0.05). Patients with grade 1-2 or 3-4 acute GVHD had increased frequency of CMV Ag positivity. (p < 0.001)
Conclusion: Cultures taken during the febrile period and more frequent use of imaging methods may allow earlier detection of infections in immunosuppressed patients who have undergone HSCT.
Keywords: Infection; Allogeneic stem cell transplantation; CMV Ag
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) represents a curative therapeutic approach for many hematological conditions. However, infections remain a leading cause of mortality and morbidity associated with high dose chemotherapy administered for allogeneic HSCT [1], during which patients experience severe neutropenia for a time period of two to three weeks. A number of factors contribute to the development of infections associated with HSCT, including neutrophil engraftment time, severity of the disease at the time of transplantation, conditioning regimens used before the transplantation, immunosuppressive treatments, infections experienced during previous neutropenia episodes, interventional procedures, indwelling catheters, hospital hygiene and the use of HEPA filter rooms [2]. Furthermore, graft versus host disease (GVHD) may also contribute to the post-transplant infection. Therefore, early identification of high-risk patients with implementation of more effective preventive and supportive strategies for reducing the rates of infection may potentially improve the survival rates [3,4]. Also, concerns have been raised regarding higher rates of infection in developing countries that may be associated with higher incidence of morbidity and mortality [5].
Recent advances in supportive treatment modalities have resulted in decreased infection frequency in these patients. In this study, our objective was to evaluate the infections in patients undergoing HSCT from related or unrelated donors at the stem cell transplantation unit of Medical park Izmir Hospital.
Materials and Methods
A total of 219 hematopoietic stem cell transplantations performed in 199 patients between November 2012 and July 2018 at the Bone Marrow Transplantation Unit, Medical Park Izmir Hospital were retrospectively evaluated via medical records. In addition to chemotherapy, all patients received prophylactic levofloxacin 500 mg q.d, acyclovir 15 mg/kg/day, fluconazole 400 mg/day p.o., and trimethoprim-sulfamethoxazole b.i.d. one week before transplantation. For infection prophylaxis, all patients were taken to isolation rooms filtered with HEPA and patient visits were restricted in the bone marrow transplant unit. All infection episodes between the neutropenic period until the development of neutrophil engraftment were recorded. Blood, urine and sputum cultures were obtained from patients with a body temperature ≥ 38 ℃.
Definitions
Neutropenia was defined as an absolute neutrophil count of <500/mm³ in peripheral blood and neutrophil engraftment was defined as the first day of two consecutive days, on which the absolute neutrophil count was >500/mm³. Neutropenic fever was defined as a body temperature greater than 38.30 C for once or a body temperature greater than 380C sustained for more than 1 hour [6]. All patients with fever underwent a physical examination; catheter, peripheral blood, urine and sputum cultures were obtained, with sampling in any suspected area. All procedures were repeated every 24 hours when fever persisted. Blood cultures and sputum cultures were plated on blood agar, EMB agar and chocolate agar (RTA, Turkey) and isolated strains were identified by the automatized Phoenix system (BD, USA). Antimicrobial susceptibility was also investigated by the automatized Phoenix system (BD, USA). Urine cultures were plated on blood agar and EMB agar (BD, USA) and isolated strains were identified by the automatized Phoenix system (BD, USA). Antimicrobial susceptibility was studied by using the automatized Phoenix system (BD, USA).
Infections during a neutropenic period were defined as follows;
i. Microbiologically documented infection if any etiological agent grows in a culture medium
ii. Clinically documented infection; if any etiological agent cannot be identified, even though symptoms and signs of a localized infection are present
iii. Fever of unknown origin; if fever is not accompanied by any symptoms or signs indicating the cause of fever and any etiological agent cannot be identified in a culture.
Empirical antibiotic therapy with meropenem and teicoplanin was administered at the onset of fever in all patients with neutropenic fever who had a central venous line. Amikacin and/or colimycin, tigecycline were added to the therapy regimen in the occurrence of a septic shock. Initiation of antifungal therapy was taken into the consideration in cases with fever persisting longer than 72 hours, or suspected fungal infection in high resolution computed tomography (HRCT) or proven fungal infection.
Diagnosis pf cytomegalovirus (CMV) antigenemia was carried out by Argene assay (Biomerieux, France). The site of infection was determined by clinical evaluation, radiography or positive culture from blood, urine, sputum, abscess, or catheter samples. Treatment was adjusted according to the infectious agent responsible for the fever, if identified. Antibiotics were discontinued 3 to 5 days after neutrophil engraftment had occurred and a fever response had been obtained.
Statistical Analysis
Data are shown as mean ± SD for normally distributed continuous variables, median (minimum-maximum) for continuous variables with skewed distribution, and frequencies for categorical variables. Pearson chi-square test was performed for the comparison of categorical variables. Means of normally distributed continuous variables were compared by ANOVA. Skew-distributed continuous variables were compared by Mann Whitney U test. Overall survival (OS) was calculated as the time elapsed from the date of diagnosis to the date of last contact or death. Cox regression analysis was used for the multivariate analysis. Statistical Package for Social Sciences (SPSS) for Windows version 15.0 (SPSS Inc., Chicago) was used for the analysis and two-sided p value of <0.05 was considered as significant.
Results
Of all participants, 72(%36.2) were female and 127 (%63.8) were male. The median age at the time of transplantation was 44 years (range: 16-77 years). Myeloablative related, non-myeloablative related, myeloablative unrelated, and non-myeloablative unrelated allogeneic stem cell transplantation was performed in 95 (43.4%), 78 (35.6), 31 (14.2%), and 15 (6.8%) patients, respectively. Peripheral stem cells were used in all transplantations. While 142 patients (64.8%) were in remission at the time of allogeneic hematopoietic stem cell transplantation, 64 (29.2%) had active disease and 13 (6%) had partial response.
The median duration of time from diagnosis to transplantation was 256 days (33-5187 days), and the median CD34 product number administered was 7.33 x 10 6/kg (1.83-34.80 x 10 6/kg) (Table 1). One-hundred and twenty patients (54.8%) had an ECOG performance status score of 1 when undergoing allogeneic-HSCT.
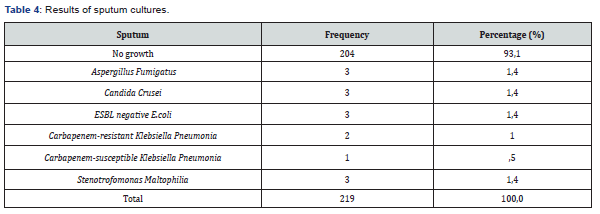
A temporary 3-way catheter was placed into the subclavian vein under the guidance of ultrasound by an interventional radiologist. Neutrophil engraftment occurred at an average of 14 days after the transplantation, while platelet engraftment occurred at an average of 23 days. Neutropenic fever occurred in 203 patients (92.7%). A treatment regimen consisting of meropenem and teicoplanin was initiated empirically at the onset of neutropenic fever. The treatment regimen was adjusted according to the infectious agent when the underlying cause of fever was identified. The cause of fever remained unknown in 20% of the patients, while 51.25% of the patients had a microbiologically documented infection and 28.75% of the patients had only clinically documented infection. Three patients were considered to have confirmed IPA and were placed on antifungal therapy; furthermore, antifungals were given to 22 other patients as a preemptive treatment (10.5%). Microbiological growth was observed in blood, urine, and sputum samples in 64 (29.2%), 29 (13.7%), and 12 (5.5%) patients, respectively (Tables 2-4). Forty-eight patients had catheter related infections, 29 patients had urinary tract infections, and 22 patients had possible, and 3 patients had documented invasive pulmonary aspergillosis. During the follow-up period, CMV Ag positivity was detected in 78 patients (35.6%), while galactomannan was positive in 24.

Twelve patients (6.03%) die due to infection prior to neutrophil engraftment. Causes of death included pneumonia due to carbapenem-resistant Klebsiella pneumonia infection in five patients, pneumonia in two, candidemia and veno-occlusive disease in one, vancomycin-resistant Enterococcus infection in one, and invasive pulmonary aspergillosis (IPA) in 3. In three of the patients who died due to carbapenem-resistant Klebsiella pneumonia infection, the organism was also resistant to tigecycline as well as colistin. All Gram-positive isolates were susceptible to glycopeptides.
Twenty percent of the carbapenem-resistant Klebsiella pneumonia strains were also resistant to colistin and tigecycline, while 80% were susceptible to colistin and/or tigecycline. Methicillin resistant Coagulase Negative Staphylococci (MRCNS) and Methicillin resistant Staphylococcus epidermidis (MRSE) were isolated from catheter and peripheral blood cultures in 26 and 14 patients, respectively, while ESBL-positive E. coli was isolated in peripheral blood cultures in three patients, and carbapenem-resistant Klebsiella pneumonia was isolated in catheter and peripheral blood cultures in nine patients. In urine cultures, E. coli, Enterococcus faecium, carbapenem-resistant Klebsiella pneumonia, carbapenem-susceptible ESBL positive Klebsiella pneumonia, and vancomycin resistant enterococci were isolated in 9, 12, 5, 7, 4, and one patient, respectively. In sputum cultures Aspergillus fumigatus, Candida krusei, ESBL (-) E. coli, carbapenem-resistant Klebsiella pneumonia, and carbapenem-susceptible ESBL positive Klebsiella pneumonia were isolated in 3, 3, 3, and 2 patients, respectively (Tables 2-4).
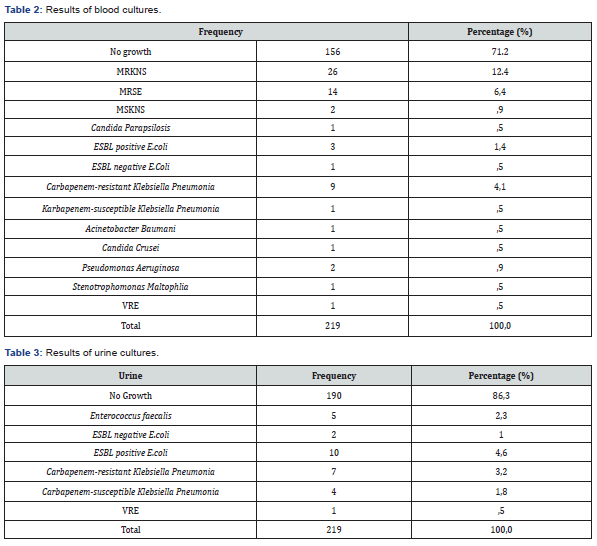
A comparison of patients with or without microbiological growth in blood samples showed no significant differences in terms of gender, age, diagnosis, type of transplantation, use of ATG, disease status at the time of transplantation, day of neutrophil and platelet engraftment, CD34 product number, presence or absence of acute or chronic GVHD, and ECOG performance status. Patients who received myeloablative busulfan-cyclophosphamide as a conditioning regimen were more likely to have positive growth in cell cultures (p < 0.018) (Table 5).
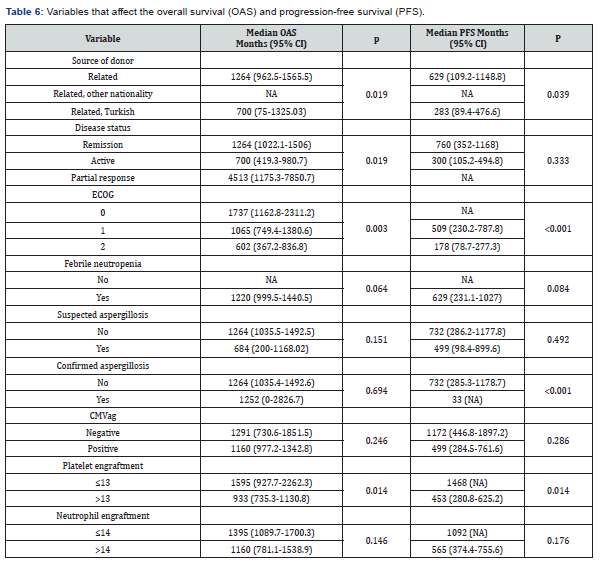
Patients with a positive sputum culture (n=12) were over 55 years of age, and had higher ECOG scores, received myeloablative conditioning regimens, were in the 2nd and 3rd remission at the time of transplantation, and had delayed platelet engrafment (p < 0.05). Also, female patients were more likely to have positive urine cultures (p <0.001) (Tables 5-8). No significant associations between the CMV Ag status of the donor and CMV Ag positivity occurring in the patient during follow up (Table 8). Patients with grade 1-2 or 3-4 acute GVHD had increased frequency of CMV Ag positivity ((Acute GVHD grade 1-2 CMV ag negative: 11.3% vs. CMV ag positive positive: 28.2%, and grade 3-4, CMV ag negative: 4.3% vs. CMV ag positive: 16.7%, p < 0.001) Also, patients with documented aspergillosis, as well as patients with positive blood or sputum cultures had shorter overall- and progression-free survival (p < 0.05).

Discussion
In patients undergoing allogeneic HSCT, infections caused by Gram-negative and Gram-positive organisms are among the main determinants of prognosis, both during transplantation and during the post-transplant period where immunosuppressive agents are administered. In a study by Kameda et al. involving patients who developed neutropenic fever following allogeneic transplantation, acute and chronic GVHD occurred more frequently, and mortality was higher [7] as compared to our study, on the other hand, patients with CMV antigenemia positivity had higher incidence of acute GVHD in the current study.
In another study, Lipari et al. reported positive blood cultures in 99 of 451 patients (21.95%) with hematological malignancy undergoing autologous and allogeneic HSCT. In that study 17% of the patients died due to infection, and Gram-negative bacteria (E. coli, 45%, Klebsiella spp. 23%, Pseudomonas spp. 11%, Acinetobacter and other bacilli 15%) were isolated in 63% of the patients, while Gram-positive cocci were isolated in 33%, and fungi were isolated in 3% [8].
In the study by Vaviov VN et al. [9] where 155 patients undergoing allogeneic transplantation were involved, a myeloablative conditioning regimen was given to 39% of the patients, and neutropenic fever developed in 63% of the subjects. Patients with mucositis had an increased frequency of neutropenic fever (69% vs. 52%, p < 0.02), and the Gram-positive cocci were the most frequently isolated organisms (45%). Among Gram-negative bacteria, Klebsiella pneumonia and Enterobacter cloacae were the most common gram-negative bacteria (24%). The rate of infection-related mortality was 2%. Neutropenic fever was more frequent among those with a more prolonged course of neutropenia, oral colonization, or invasive fungal infections. On the other hand, in our study neutropenic fever developed in 203 patients (92.7%) during transplantation, and causative organisms were isolated in blood cultures in 64 (29.2%) subjects. Gram positive organisms were grown in 44 patients, gram negative organisms in 18 patients, and candida spp. in 2 patients. Accordingly, gram positive bacteria were more common in our participants. Twelve patients (6.03%) died due to infections in the first 30-day period. At 2 years of follow-up, CMV Ag positivity was present in 78 subjects (35.6%), while 24 had galactomannan positivity during the neutropenic episode at the course of transplantation.
In a study from Iran by Rafayi et al., 20% of the AML patients were CMV Ag positive during the follow-up after allogeneic HSCT [10]. In our cohort, the higher rate of patients with positive CMV antigenemia may be accounted for by the inclusion of patients with unrelated donors in whom more intense immunosuppressive therapy was administered. In the current study, 203 patients (92.7%) developed fever, and the cause of the fever could not be determined in 32.9%, while only 20% had clinically documented infections and 47.1% had microbiologically documented infections. As compared to studies from Western countries where a NPA incidence of 60 to 90% was reported for this patient population, the corresponding figures were 82.5% in India [5], and 98% in Iran [10]. Three patients were considered to have proven IPA and started on antifungals and antifungals were given to 22 other patients as a preemptive treatment (11.4%) However, the incidence of fungal infection varies from 4% to 30 in different transplant centers [11,12].
Gram-positive microorganisms were detected in most of the microbiologically documented infections. This result is closely linked to the use of central venous lines [13] and suggests inadequacy of sterile barriers used in transplantations. Therefore, the use of antimicrobial-coated catheters has been suggested and this type of catheters were found to be associated with a reduced incidence of infections in a study conducted in intensive care patients [14]. In our center, central venous lines are placed by the same team of interventional radiologists and sterile catheter dressings are replaced every 48 hours. Higher rates of microbiologically documented infections in our study might reflect the efficiency of our Microbiology Laboratory as well as pointing out to the fact that catheter, urine, sputum, and peripheral blood samples for cultures were collected every 24 hours beginning from the onset of febrile neutropenia and continuing throughout the febrile period. Higher detection rates for documented infections in the present study may be related to the above-mentioned factors, and to the more frequent use of imaging modalities. In addition to preventive measures, early detection of the causative organisms bears crucial significance for optimum treatment and improved prognosis in this patient population.
Conclusion
Infections caused by Gram-negative or Gram-positive microorganisms have a particular impact on the prognosis of immunocompromised patients. In our department, infections do not seem to pose a significant mortality risk in patients undergoing allogeneic stem cell transplantation. In addition to preventive measures, early detection of the causative organisms bears crucial significance for optimum treatment and improved prognosis in this patient population.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Petersen SL (2007) Alloreactivity as therapeutic principle in the treatment of hematologic malignancies Studies of clinical and immunologic aspects of allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning. Dan Med Bull 54(2): 112–139.
- Sanchez-Guijo FM, Orfao A, Canizo MC (2012) Bone Marrow Transplantation Extends Its Scope. Adv Exp Med Biol 741: 121–134.
- Hoyle C, Goldman JM (1994) Life-threatening infections occurring more than 3 months after BMT 18 UK Bone Marrow Transplant Teams. Bone Marrow Transplantation 14(2): 247–252.
- Majhail NS, Rizzo JD, Lee SJ, Aljurf M, Atsuta Y, et al. (2012) Recommended Screening and Preventive Practices for Long-Term Survivors after Hematopoietic Cell,Hematol Oncol Stem Cell Ther 5: 1–30.
- George B, Mathews V, Srivastava A, Chandy M (2004) Infections among allogeneic bone marrow transplant recipients in India. Bone Marrow Transplantation 33(3): 311–315.
- Hughes WT, Armstrong D, Bodey GP, Brown AE, Edwards JE, et al. (1997) 1997 guidelines for he use of antimicrobial agents in neutropenic patients with unexplained fever. Infectious Diseases Society of America. Clin Infect Dis 25(3): 551–573.
- Kameda K, Kimura SI, Misaki Y, Yoshimura K, Gomyo A, et al. (2019) Associations between febrile neutropenia-related parameters and the risk of acute GVHD or non-relapse mortality after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant 54(5): 707-716.
- Lipari FG, Zarate AH, Garcia JJ, Basquiera AL, Caeiro JP (2017) Bloodstream infection in patients receiving hematopoietic stem cell transplant. Seven years of experience with adults and children. Rev Chilena Infectol 34(6): 535-538.
- Vavilov VN, Averyanova MY, Bondarenko SN, Stancheva NV, Zubarovskaya LS, et al. (2015) Bacterial infections in the early period after allogeneic bone marrow transplantation. Ter Arkh 87(7): 88-93.
- R Safayi, F Shahi, M Ghalamkari, M Mirzania, M Khatuni, et al. (2018) A Survey of Infection in Allogenic Hematopoietic Stem Cell Transplantation in Patients with Acute Myeloid Leukemia Int J Organ Transplant Med 9(3): 112–116.
- Ninin E, Milpied N, Moreau P, Andre-Richet B, Morineau N, et al. (2001) Study of bacterial, viral and fungal infections in adult recipients of bone marrow transplants. Clin Infect Dis 33(1): 41–47.
- Hovi L, Saarinen-Pihkala UM, Vettenranta K, Saxen H (2000) Invasive fungal infections in pediatric bone marrow transplant recipients: single center experience of 10 years. Bone Marrow Transplant 26(9): 999–1004.
- Gil L, Styczynski J, Komarnicki M (2007) Infectious Complication in 314 Patients after High-Dose Therapy and Autologous Hematopoietic Stem Cell Transplantation: Risk Factors Analysis and Outcome, Infection 35(6): 421–427.
- Winston DJ, Gale RP, Meyer DV, Young LS (1979) Infectious complications of human bone marrow transplantation. Medicine(Baltimore) 58(1): 1–31.






























