- Research Article
- Abstract
- Introduction
- Material and methods
- Results
- Discussion
- Conclusion
- Compliance with ethical standards
- Ethics approval and consent to participate
- Consent for publication
- Availability of data and materials
- Competing Interests
- Funding information
- Authors’ contributions
- Acknowledgement
- References
Luteolin Induced Apoptosis and Blockage of P-Glycoprotein in Human Sarcoma Cell Lines
Dorra El Gueder1,2*, Aida Elahmar2, Zaineb Dhaouefi2, Imene Ben Toumia2, Fairouz Sioud2, Aicha Sassi2, Jose Luis3 and Leila Chekir Ghedira2
1Faculty of Sciences of Tunis, University of Tunis El Manar, 2092 Tunis, Tunisia
2 Faculty of Dental Medicine, Unity of Bioactive and Natural Substances and Biotechnology UR17ES49, University of Monastir, Avicenne Street, 5000 Monastir, Tunisia
3 CNRS, Institut de Neurophysiopathologie, Aix Marseille University, Marseille, France
Submission: January 15, 2021; Published: May 20, 2021
*Corresponding Address:Dorra El Gueder, Faculty of Sciences of Tunis & Faculty of Dental Medicine, University of Tunis El Manar, Unity of Bioactive and Natural Substances and Biotechnology UR17ES49, University of Monastir, Avicenne Street, 5000 Monastir, Tunisia
How to cite this article:Dorra El G, Aida E, Zaineb D, Imene B T, Fairouz S, et al. Luteolin Induced Apoptosis and Blockage of P-Glycoprotein in Human Sarcoma Cell Lines. Canc Therapy & Oncol Int J. 2021; 18(5): 555997.DOI: 10.19080/CTOIJ.2021.18.555997
- Research Article
- Abstract
- Introduction
- Material and methods
- Results
- Discussion
- Conclusion
- Compliance with ethical standards
- Ethics approval and consent to participate
- Consent for publication
- Availability of data and materials
- Competing Interests
- Funding information
- Authors’ contributions
- Acknowledgement
- References
Abstract
Resistance to chemotherapy is the main problem in the treatment of cancer. In many cases, this resistance is due to the over-expression of the P-glycoprotein (pg-p) that pumped out therapeutic drugs. Luteolin, a natural flavonoïd, is abundant in our daily diet and has many pharmacological properties. A large body of evidence shows that luteolin is a potent anti-cancer agent. In the present study, cell growth was assessed by MTT assay. Reactive oxygen species generation was measured with DCFH-DA staining; mitochondrial membrane potential was tested with rhodamine-123 using fluorometer, cell apoptosis was detected with flow cytometry and acridine orange staining, DNA damage was visualized by comet test and salting out method. Finally, drugs transporter activity of was measured by flow cytometry. Results indicated that luteolin could significantly inhibit growth of human sarcoma cells. In addition, analysis of various parameters indicated that luteolin induced apoptosis by generation of reactive oxygen species, DNA damage and decrease in mitochondrial membrane potential. We also observed that luteolin inhibited cell cycle progression at sub G1 phase and prevented cells to entry into S phase. Moreover, we suggested that luteolin is able to affect function of drug transporters by blockage of P-glycoprotein pump whose daunorubicin accumulation. Finally, our findings reveal luteolin as a pluripotent anti-tumor agent in multidrug resistant cancer cells MES-SA/Dx5 that can be considered for new treatment approaches
Keywords: Cell cycle; Daunorubicin; DNA damage; Multidrug resistant; P-glycoprotein; Proliferation
- Research Article
- Abstract
- Introduction
- Material and methods
- Results
- Discussion
- Conclusion
- Compliance with ethical standards
- Ethics approval and consent to participate
- Consent for publication
- Availability of data and materials
- Competing Interests
- Funding information
- Authors’ contributions
- Acknowledgement
- References
Introduction
Multidrug resistance (MDR) is the main issue encountered in cancer handling by chemotherapy. Recently, it appears that pgp is potentially an MDR mediator [1]. The major cause of this resistance cancer cells is the overexpression of P-glycoprotein (pg-p) that pumps out therapeutic drugs such as anthracyclines (daunorubicin and doxorubicin). This results in a decrease in the intracellular accumulation of drugs and thus reduces the efficacy of treatment [2,3]. P-glycoprotein is a glycosylated membrane protein belonging to ABC transporters family [4] that is encoded by the ABCB1 gene [5].
The discovery of pg-p inhibitors able to reverse the MDR in cancer cells is thus an interesting avenue to improve chemotherapy. Fumitremorgin C (FTC), a natural fungal product, is thus a selective inhibitor of breast cancer resistance protein and can also trigger the transition from cell cycle arrest at G2/M [6]. Other studies have shown the presence of macro cyclic diterpenes which are a new compound of potent pgp reversal agents [6]. On the other hand, [7] repoted that a low dose of cyclosporine A could inhibit the function of pg-p in vitro without altering its expression in multidrug-resistant leukemia cells (K562).
Other various natural molecules, such as luteoline, have received a lot of attention in cancer prevention because of their anticancer properties. Luteolin, a flavonoid found in many vegetables and fruits, has been used in traditional Chinese medicine to heal many illnesses. This natural product displays various properties including anti-inflammatory, antioxidant and anti-tumor activities, and it has also been suggested as a powerful cancer prevention agent [8]. Many researchers have reported that luteolin triggers apoptosis in a various cellular model by targeting the apoptosis signaling pathways [9-11]. Moreover, luteolin induced cell cycle arrest by increasing the population of cells in the S phase and decreasing the cell viability of the NCI-ADR/RES and MCF-7/Mito cell lines [12]. Mitochondria play an essential role in apoptosis, thereby, the pro-apoptotic proteins has an important function in reducing the potential of mitochondrial membrane (ΔΨm) and thus the release of cytochrome C from mitochondria and therefore leads to accelerate apoptosis [13].
Several studies indicate that the increase of oxidative stress may sensitize cancer cells to treatments [14] because they have an unbalanced redox status compared to normal cells [15]. At high concentrations, luteolin is also a reactive oxygen species (ROS)-generating agent in prolonged incubation. In addition, other studies have shown that luteolin treatment causes DNA fragmentation [16], chromatin condensation and cell shrinkage [17]. In this present review, we further investigated the anticancer impact of luteolin, and we report its capacity to reverse daunorubicin resistance in a human uterus sarcoma drugresistant cell line that overexpresses pgp.
- Research Article
- Abstract
- Introduction
- Material and methods
- Results
- Discussion
- Conclusion
- Compliance with ethical standards
- Ethics approval and consent to participate
- Consent for publication
- Availability of data and materials
- Competing Interests
- Funding information
- Authors’ contributions
- Acknowledgement
- References
Material and methods
Chemicals and Reagents
Luteolin was bought from Sigma Aldrich (Genay, France) and was of the highest purity available. This compound was dissolved in dimethyl sulfoxide (DMSO) and then diluted with buffer (1:199, v/v). Trypsin, penicillin, streptomycin, RPMI-1640 medium, and fetal bovine serum (FBS) were bought from Gibco⁄BRL (Scotland, UK). 3-(4, 5- dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT), annexin V apoptosis detection kit, propidium iodide (PI), daunorubicin and cyclosporin were bought from Sigma-Aldrich (Genay, France). Ribonuclease A (RNase), proteinase K, DMSO were procured from Sigma-Aldrich (Genay, France), 2′,7′- dichlorofluorescin diacetate (DCFH-DA) was obtained from Fluka Steinheim Germany), agarose from (Lonza, Rockland, ME, USA), Tris–HCl (Biobasic, Canada) and sodium sarcosinate from (Biomedical, Germany).
Cell culture
The multiple drug-resistant cell line MES-SA/Dx5 (CRL-1977; ATCC, Manassas, VA) was established from the human sarcoma cell line MES-SA (CRL-1976; ATCC) in the presence of increased doxorubicin concentrations [18]. Cell lines were cultured in RPMI- 1640, containing 10% (v/v) fetal bovine serum, 20 mM glutamine and 100 units/ml penicillin and 100 μg/ml streptomycin at 37°C and 5% CO2 incubator and the culture medium was renewed every 2–3 days.
Cell viability MTT assay
To establish the effect of luteolin on MES-SA/Dx5 cells, the cytotoxicity toward resistant tumor cells was before all else determined by MTT assay. Cells were plated at an approximate density of 3× 103 cells/well in a 96-well plate. After 24 h incubation, cells were intoxicated with different concentrations of luteolin for 48 h. Cell viability was evaluated by 3-(4,5-cimethylthiazol-2-yl)- 2,5-diphenyl tetrazolium bromide (MTT) assays as previously described [19].
Measurement of intracellular reactive oxygen species (ROS)
Intracellular generation of ROS was measured using DCFHDA reagent. Cells were seeded into 96-well plates at density of 5×104 cell/well. After 24 h, cells were loaded with 5 μM DCFH-DA (25 μM) and incubated with different concentrations of luteolin. The ROS generated were determined after 2 h of incubation by measuring the fluorescent DCF in a plate-reader at an excitation wavelength of 485 nm and emission wavelength of 525 nm.
Measurement of mitochondrial membrane potential
The uptake of the cationic fluorescent dye rhodamine-123, a positively charged molecule which accumulates within mitochondria in inverse proportion to Δψm according to the Nernst equation, was applied for the evaluation of mitochondrial membrane potential [20]. Briefly, cells were seeded in 96-well culture plates and intoxicated with luteolin for 48 h. Cells were then carefully rinsed with phosphate-buffered saline pH7.4 (PBS), and 100 μl of rhodamine-123 (5μM) in PBS was added in the plates. Cells were incubated for 1 h. After that, the supernatant PBS (containing unuptaked rhodamine-123) was removed and replaced by fresh PBS. After 30 min, ethanol (100%) was added and the uptake of rhodamine-123 was measured using a fluorescence microplate reader (BioTek, Winooski, USA) with 538-nm emission and 485-nm excitation filters.
Analyse of Cell cycle using flow cytometry
5 × 105 cells were seeded into 6-well culture plates and incubated for 24 h. Cells were intoxicated with various concentrations of luteolin for 48 h, trypsinized and washed twice in PBS. After that, cells were added by ice-cold absolute ethanol for 30 min, treated with ribonuclease A (10 mg/ml) for 30 min at 4°C and stained with 50 μl propidium iodide (1 mg/ml) for 10 min. Cell cycle analysis was conducted using FACS system (Beckman Coulter, Switzerland). Percentages of cells in each phase of the cell cycle were calculated.
Analysis of apoptosis
To detect and quantify apoptotic cells, annexin-V-FITC was combined with propidium iodide to allow the distinction between early and late apoptotic, necrotic and live cells. 5 × 105 cells were seeded into 6-well culture plates and incubated for 24 h before treatment with different concentrations of luteolin for 48 h. Cells were then trypsinized, washed with PBS and suspended with 500 μl of binding buffer containing 5 μl of annexin V-FITC and 5 μl of propidium iodide (PI). Cells were incubated for 15 min in the dark and subjected to flow cytometry using FACS system (Beckman Coulter, Switzerland).
Comet Assay
DNA damages in MES-SA/Dx5 were detected by using the comet assay as explained by [21]. Before each experiment, frosted microscope slides were precoated with normal agarose 1% and left at room temperature to enable the agarose to dry. 5×105 cells were seeded into 6-well culture plates and incubated for 24 h before treatment with different concentrations of luteolin. After 48h incubation, cells were trypsined and washed twice in PBS. The cell dilution (5 × 105 cells in 60 μl) was mixed with 60 μl of low-melting-point agarose and the mixture was spread onto a precoated slide and covered with a coverslip. After 10 min on ice, the coverslip was gently removed, and the slides were immersed in a lysis solution (2.5 M NaCl, 100 mM EDTA), 10 mM Tris–HCl, 1% sodium sarcosinate pH 10, 1% of Triton X-100, and 10% DMSO. After 1 h at 4 °C in the dark, slides were transferred into the electrophoresis buffer (NaOH 10 N and EDTA 200 mM in deionized water) for 20 min at room temperature in the dark. Electrophoresis was carried out for 15 min at 25 V and 300 mA. Finally, the slides were gently rinsed three times for 5 min with a neutralizing solution (0.4 M Tris–HCl, pH 7.5). Staining of DNA was accomplished by ethidium bromide solution (20μg/ ml) in PBS. The slides were visualized using an epifluorescence microscope (Zeiss Axioskop 20, Carl Zeiss, Microscope Division, Oberkochen, Germany).
Comet assay quantification
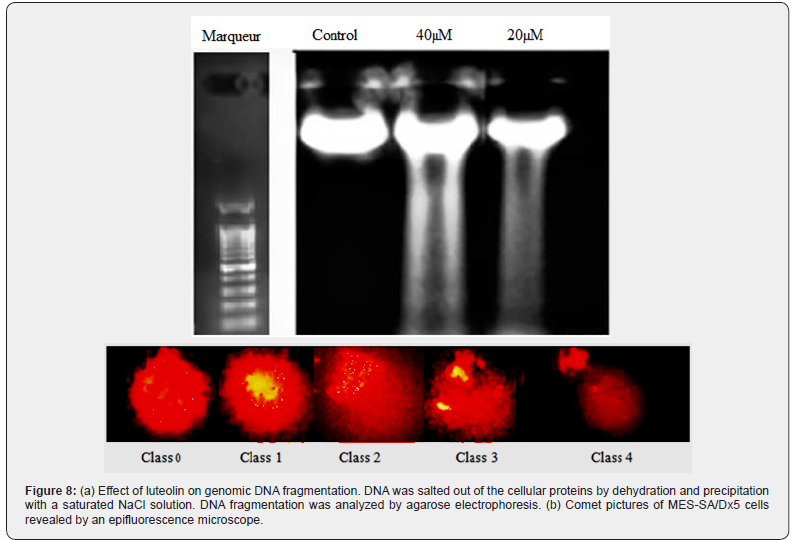
Depending on the relative intensity of fluorescence in the tail, a total of 100 comets were visually noted on each slide and classified as belonging to one of five classes as illustrated in Figure 8b. As previously described by [22], the value of each category of comet ranges from 0 (undamaged) to 4 (maximally damaged) as shown in Figure 8b. The total DNA damage score is calculated by the following equation: Total DNA Damage (TDD) = (percentage of cells in class 0 × 0) + (percentage of cells in class 1 × 1) + (percentage of cells in class 2 × 2) + (percentage of cells in class 3 × 3) + (percentage of cells in class 4 × 4).
Acridine orange and ethidium bromide staining assay
Cells (5×105) were seeded into 6-well culture plates and incubated at 37°C for 24 h. After 48h of treatment with different concentrations of luteolin, cells were trypsinized and washed twice in PBS. Cells were resuspended in 1 ml PBS containing 10 μg acridine orange (AO) and 10 μg ethidium bromide, and incubated at room temperature for 10 min. Finally, the cells were washed twice with PBS and placed on glass slide, and 100 cells were counted using a fluorescent microscope (Zeiss, Oberkochen, Germany) and the apoptotic chromatin condensation was scored. Cells stained orange are apoptotic cells (nuclei fragmented), those stained red are necrotic cells (uncondensed and dispersed chromatin), and those stained green are healthy cells (condensed chromatin).
Analysis of DNA fragmentation
A fast, safe and inexpensive method was developed to simplify the deproteinization process. This DNA extraction method involves salting out of cellular proteins by dehydration and precipitation with saturated NaCl solution. DNA was extracted as described by [23]. Cells were seeded into 6-well microtiter plates (at 5×105 cells/well; 2 ml) and incubated at 37 °C in the 5% CO2 incubator. After 24 h, serial dilutions of luteolin were added to wells and the plates were incubated a further 48 h. Cells from each well were harvested, washed with PBS, and resuspended in 1.5 ml lysis buffer (10 % SDS; 2 M Tris-HCI pH 7.5; 3 M NaCI; 0.4 M EDTA pH 8; 10 mg/ml proteinase K) for 2h at 56°C.
After that, ribonuclease A (10 mg/ml) was added to each sample at 65°C for 1 h and the cellular proteins were salted out by dehydration with saturated NaCI (6 M). The DNA was precipitated with absolute ethanol, washed twice with ethanol 70% and dissolved in free H2O before running in a 1% agarose gel electrophoresis containing ethidium bromide (1 mg/ml) at 80 V and 200 mA. Approximately, 5μl DNA was loaded in each well and visualized under UV light and photographed.
Evaluation of P-glycoprotein drug efflux modulation
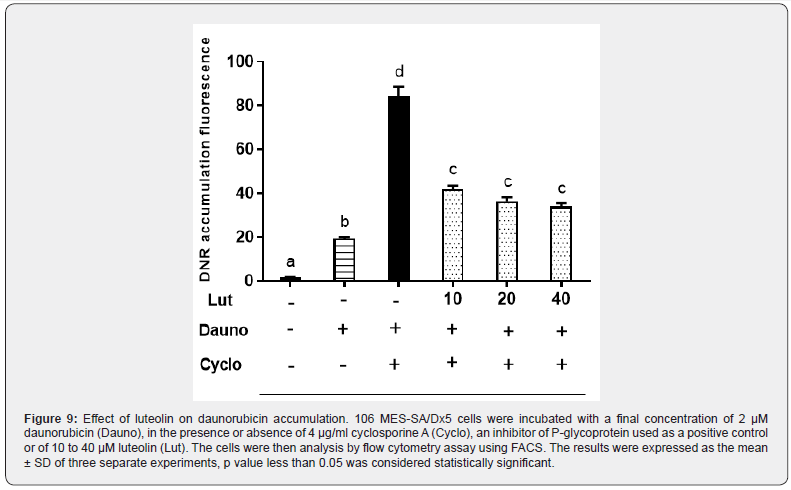
As described by [24], luteolin was tested on the MES-SA/ Dx5 MDR cell line overexpressing P-glycoprotein, 106 MES-SA/ Dx5 MDR cells that express high levels of P-glycoprotein were incubated for 1 h at 37°C in 1 ml RPMI 1640 medium without antibiotic containing a final concentration of 2 μM daunorubicin (DNR), in the presence or absence of 4 μg/ml cyclosporine A (CsA), an inhibitor of P-glycoprotein used as a positive control. Cells were then washed twice with ice-cold PBS and kept on ice until analysis by flow cytometry assay using FACS system (Beckman Coulter, Switzerland). Luteolin were tested at concentrations of 20, 10 and 5 μM. In the presence or absence of luteolin, its ability to inhibit Pgp-mediated drug efflux was quantified by comparing the intracellular fluorescence of DNR and compare it with values recorded with the positive control (CsA).
Statistical analysis
Each experiment was carried out at least three different times, with essentially identical results. Apoptosis analysis was carried out twice. Statistical analysis was done using the SPSS 20.0 statistic software via test ANOVA one-way analysis. The graphics were performed using GraphPad Prism software. The results were expressed as the mean with ±SEM. p value was considered statistically significant when it was less than 0.05.
- Research Article
- Abstract
- Introduction
- Material and methods
- Results
- Discussion
- Conclusion
- Compliance with ethical standards
- Ethics approval and consent to participate
- Consent for publication
- Availability of data and materials
- Competing Interests
- Funding information
- Authors’ contributions
- Acknowledgement
- References
Results
Luteolin inhibits cell viability
In order the effect of luteolin on the viability of human sarcoma cancer cells, MES-SA-Dx5 cells were treated with different concentrations of luteolin (20, 40, 80, 160 μM) for 48 hours and the cell viability was evaluated by the MTT assay. As shown in Figure 1, luteolin induces cell death in a dose-dependent manner, and the IC50 value is estimated to be 20 μM ± 5 μM.
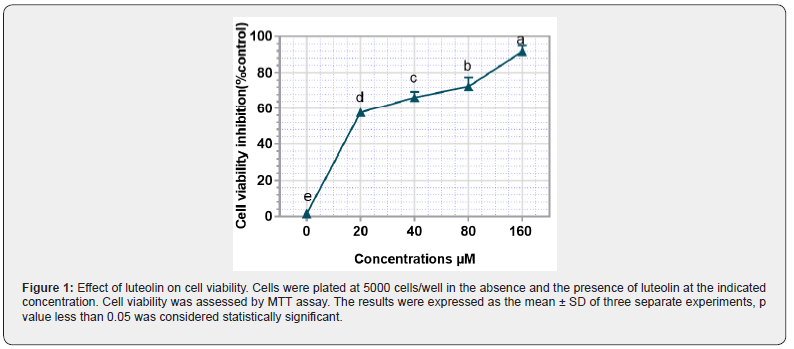
Luteolin promotes ROS production
Effect of luteolin on production of ROS was determined by measuring the increase of fluorescence upon hydrolysis of DCFHDA and its oxidation by ROS. As illustrated in Figure 2, untreated MES-SA/Dx5 cells generated ROS because of normal metabolic activity. However, luteolin at 160 μM significantly increased ROS production, suggesting that luteolin is an ROS generating agent at high concentration.
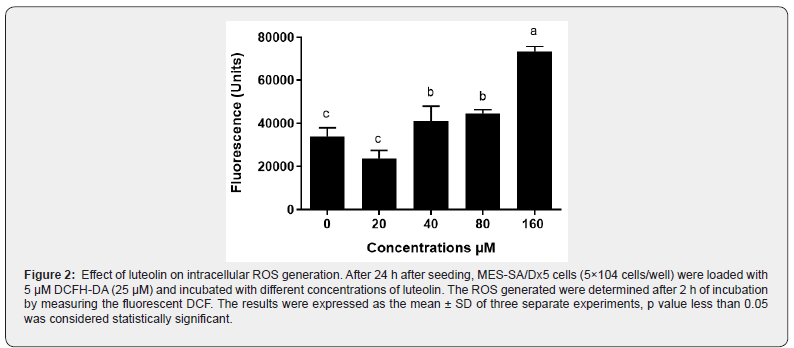
Luteolin induces cell cycle arrest
To study the impact of luteolin on cell cycle arrest, we applied fluorescence activated cell sorting (FACS) analysis by flow cytometry (Beckman coulter, Switzerland) to determine the number of cells in each stage of cell cycle. Figure 3 shows that the number of cells in sub G1 peak increased significantly from 1.5% (control) to 40.5% at 40 μM luteolin in a concentration-dependent manner. Concomitantly, the number of cells in G0G1 peak decreased gradually from 00% to 00%. No significant difference in S and G2M phases could be observed upon treatment with luteolin. These results show that luteolin provokes an accumulation in sub G1 population with simultaneous decrease in G0G1 phase.
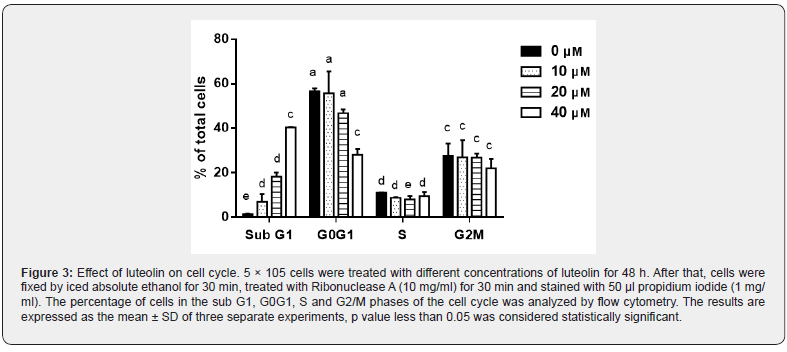
Luteolin disrupts the mitochondrial potential
The decrease of mitochondrial membrane potential (ΔΨm) causes the perturbation of the mitochondrial outer membrane. As shown in Figure 4, a decrease in mitochondrial potential was detected after 1h of incubation with luteolin in a dose-dependent manner. This data indicates that luteolin can change mitochondrial membrane potential, suggesting that it may be involved in an apoptotic mechanism via the mitochondrial pathway in MES-SA/ Dx5 cells.
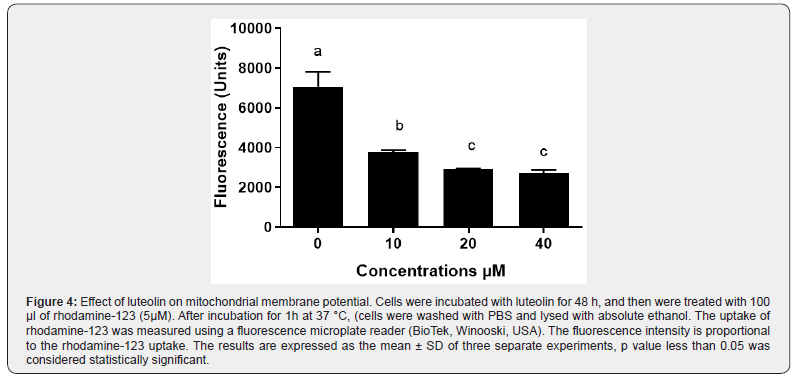
Luteolin induces apoptosis in human sarcoma cancer cells
Cell apoptosis is known as a normal physiologic mechanism of controlled cell death to keep a stable homeostasis. To determine the effect of luteolin on apoptosis, annexin V-FITC/PI double staining assay was performed on MES-SA/Dx5 cells. As summarized in Figure 5 upon treatment with luteolin, the population of cells in early and late apoptosis increased, while the population of viable cells decreased. In addition, acridine orange staining results also proved that luteolin could induce apoptosis of MES-SA/Dx5 (Figure 6a). Control cell nuclei were stained only with a green fluorescence, while, in the treated cells with luteolin, nuclei were stained with orange fluorescence in majority of cells (Figure 6b). These findings confirmed that luteolin increased the percentage of apoptotic cells in a concentration dependent manner.
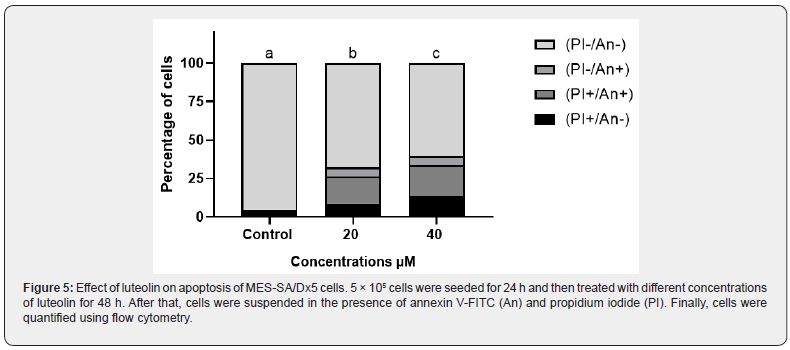
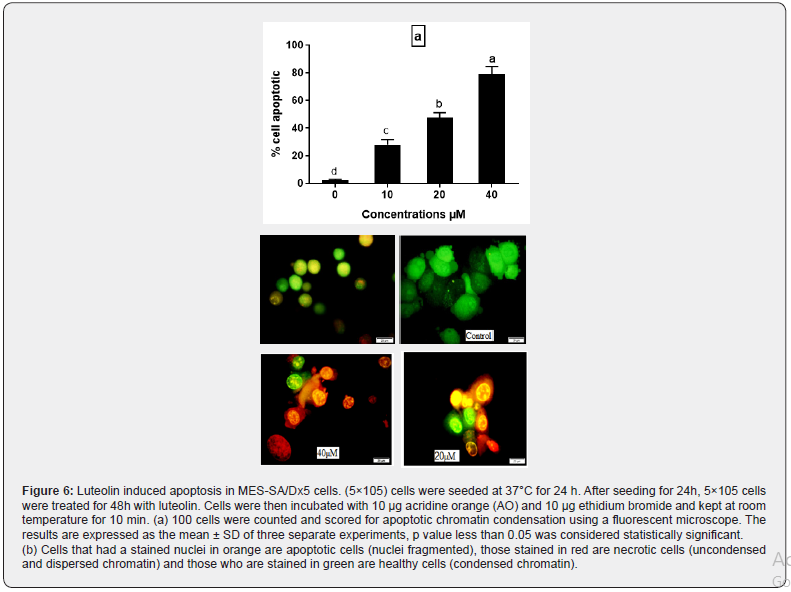
Luteolin induces DNA damage in human sarcoma cancer cells
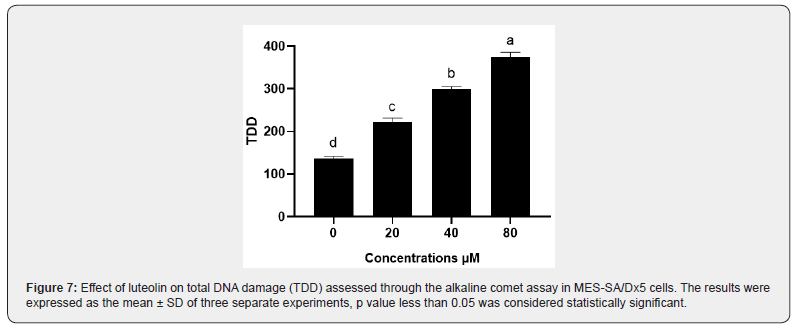
Total DNA damage was first estimated by the alkaline comet assay. The total score of DNA damage is shown in Figure 7. We observed that in MES-SA/Dx5 cells treated with different doses of luteolin, total DNA damage increased in a dependent manner compared with the control group. DNA fragmentation is a hallmark of apoptosis. Therefore, we secondly treated MES-SA/ Dx5 cells with 20 or 40 μM of luteolin for 48h and analyzed the degree of DNA damage (Figure 8a). In the presence of of luteolin, DNA fragmentation was clearly visible for both concentrations of luteolin. Altogether, these data confirm that luteolin triggers apoptosis in MES-SA/Dx5 cells.
Luteolin is an inhibitor of P-glycoprotein
The multidrug resistant cancer cells MES-SA/Dx5 were chosen to estimate the effect of luteolin on p-gp function. The transport activity of p-gp was measured by the intracellular accumulation of fluorescent substrate (daunorubicin). The findings are presented in Figure 9. The inhibition of p-gp by cyclosporine A results in a strong increase in daunorubicin accumulation. When the experiment was performed in the presence of luteolin, we also observed an accumulation, although lower, of daunorubicin. The fluorescent substrate accumulates by about 2-fold in the presence of luteolin compared to control cells with only daunorubicin.
- Research Article
- Abstract
- Introduction
- Material and methods
- Results
- Discussion
- Conclusion
- Compliance with ethical standards
- Ethics approval and consent to participate
- Consent for publication
- Availability of data and materials
- Competing Interests
- Funding information
- Authors’ contributions
- Acknowledgement
- References
Discussion
Many plants are used in traditional medicine due to their antitumor activity [25]. Luteolin is a flavonoid found in vegetables, fruits and traditional Chinese herbal medicines. It has been shown to have anticancer activity against a variety of cancer cells [26]. The result prove that luteolin inhibits cell proliferation in a dosedependent manner, which is in agreement with many studies showing that luteolin prevents the growth of MDA-MB-231 cells [27]. It is reported that luteolin can reduce the cell viability of human glioblastoma [28], thyroid cancer [29], lung adenocarcinoma [30], drug-resistant ovarian cancer [31], esophageal squamous carcinoma [32] and melanoma [33] in a dose dependent manner. Previous studies have shown that luteolin is an oxidizing agent that generates ROS. These results indicate that luteolin blocks the cell cycle and provoke apoptosis as well as it causes DNA damage. It also activates the signaling pathways ATR, Chk2 and p53, inhibit the NF-kB signaling pathway, activation of the p38 pathway and decrease anti-apoptotic proteins in MCF-7 / MitoR [34].
Further research has shown that treatment with luteolin can significantly increase ROS levels, leading to endoplasmic reticulum stress and mitochondrial dysfunction in glioblastoma cells U251 MG and U87 MG [35]. Other researchers have shown that mitochondria play an important role in regulating apoptosis [36]. Excessive generation of ROS causes cellular stress and alteration of membrane proteins that induces a mitochondrial dysfunction and loss of mitochondrial membrane potential (△ψm) resulting in apoptosis [37]. In this etude, we observed that the mitochondrial membrane potential of MES-SA / Dx5 cells decreased after luteolin treatment. Another result confirmed that luteolin has a good ability to change the potential of the mitochondrial membrane and stimulate the cytochrome c released in the cytosol of HL-60 cells [38]. Other findings proved that with elevated concentration of luteolin, the potential mitochondrial membrane was reduced [32].
On the other hand, these data showed that luteolin caused significant DNA damage, indicating that this flavonoid has the ability to trigger apoptosis in MES-SA / Dx5 cancer cells. Recently it has been suggested that luteolin induce an intense DNA fragmentation in MCF-7/MitoR cells [34] and HL-60 [38]. The impact of luteolin on DNA fragmentation is a characteristic of apoptosis. Numerous studies have shown that luteolin causes apoptosis in many cancer cells, like as human cervical cancer cells [39] esophageal carcinoma cells [32], colorectal cancer cells [40]. It has also been demonstrated that treatment of A375 cells with high luteolin concentrations can significantly increase the rate of apoptotic cells [41]. According to a previous studies, luteolin can cause cell cycle arrest in the G2 / M phase and cause apoptosis through the mitochondrial pathway in the cell lines EC1 and KYSE450 [32]. On the contrary, several other studies report that luteolin may promote cell cycle arrest differently in various types of cancer cell lines, either in G1/S or G2/M block. Moreover, luteoline may disrupt the cell cycle progression of MCF-7 cells in sub-G1 and G1 phases [42], In this study, we observed that luteolin treatment led to accumulation of MES-SA/Dx5 cells in sub G1 phase with a little decrease in G0/G1 phase. Previously, luteolin was reported to cause cell cycle blockage and cell accumulation in the G1 phase and not to enter the S phase of human hepatocellular carcinoma cells [43].
In addition, the result of flow cytometry showed that the increasing level of cells blocked in G2 / M phase was dosedependent in the human gastric cancer AGS cell line [44]. More than 90% of patients with metastatic cancer have a treatment failure due to their resistance to chemotherapeutic drugs. This phenomenon is explained by the enormous efflux of the chemotherapeutic agent due to the over-expression of the pgp pump. This study indicates the ability of luteolin to inhibit the pgp pump. However, this result is consistent with other results that have shown that natural compounds are characterized by their anticancer activities and can also make cancer cells more resistant to chemotherapy treatment by modulating Pgp activity [45].
- Research Article
- Abstract
- Introduction
- Material and methods
- Results
- Discussion
- Conclusion
- Compliance with ethical standards
- Ethics approval and consent to participate
- Consent for publication
- Availability of data and materials
- Competing Interests
- Funding information
- Authors’ contributions
- Acknowledgement
- References
Conclusion
In conclusion, this study shows that luteolin is a natural flavonoid able to inhibit proliferation of MES-SA / Dx5 cancer cells, and to induce apoptosis through the membrane mitochondrial potentiel decline and fragmentation of DNA, as well as it has the power to block the function of drug transporters. These findings prove that luteolin can be considered as an anti-cancer agent that can be used in the treatment of human uterine cancer. However, the actual clinical application of luteolin needs to be further investigated by extensive in vivo testing.
- Research Article
- Abstract
- Introduction
- Material and methods
- Results
- Discussion
- Conclusion
- Compliance with ethical standards
- Ethics approval and consent to participate
- Consent for publication
- Availability of data and materials
- Competing Interests
- Funding information
- Authors’ contributions
- Acknowledgement
- References
Compliance with ethical standards
The authors declares no conflict of interest.
- Research Article
- Abstract
- Introduction
- Material and methods
- Results
- Discussion
- Conclusion
- Compliance with ethical standards
- Ethics approval and consent to participate
- Consent for publication
- Availability of data and materials
- Competing Interests
- Funding information
- Authors’ contributions
- Acknowledgement
- References
Ethics approval and consent to participate
“Not applicable”.
- Research Article
- Abstract
- Introduction
- Material and methods
- Results
- Discussion
- Conclusion
- Compliance with ethical standards
- Ethics approval and consent to participate
- Consent for publication
- Availability of data and materials
- Competing Interests
- Funding information
- Authors’ contributions
- Acknowledgement
- References
Consent for publication
“Not applicable”.
- Research Article
- Abstract
- Introduction
- Material and methods
- Results
- Discussion
- Conclusion
- Compliance with ethical standards
- Ethics approval and consent to participate
- Consent for publication
- Availability of data and materials
- Competing Interests
- Funding information
- Authors’ contributions
- Acknowledgement
- References
Availability of data and materials
All data generated or analysed during this study are included in this published article.
- Research Article
- Abstract
- Introduction
- Material and methods
- Results
- Discussion
- Conclusion
- Compliance with ethical standards
- Ethics approval and consent to participate
- Consent for publication
- Availability of data and materials
- Competing Interests
- Funding information
- Authors’ contributions
- Acknowledgement
- References
Competing Interests
The authors declare that they have no competing interests.
- Research Article
- Abstract
- Introduction
- Material and methods
- Results
- Discussion
- Conclusion
- Compliance with ethical standards
- Ethics approval and consent to participate
- Consent for publication
- Availability of data and materials
- Competing Interests
- Funding information
- Authors’ contributions
- Acknowledgement
- References
Funding information
This research was supported by « Ministry of Higher Education and Scientific Research » for providing financial support.
- Research Article
- Abstract
- Introduction
- Material and methods
- Results
- Discussion
- Conclusion
- Compliance with ethical standards
- Ethics approval and consent to participate
- Consent for publication
- Availability of data and materials
- Competing Interests
- Funding information
- Authors’ contributions
- Acknowledgement
- References
Authors’ contributions
Dorra El Gueder: Responsible for conceptual design, testing and data collection, analysis and data interpretation, and drafted the manuscript.
Aida Elahmar. Zaineb Dhaouefi. Imene Ben Toumia. Fairouz Sioud. Aicha Sassi. José Luis: was involved in drafting the manuscript.
Leila Chekir Ghedira: Contributed to the concept and revised it for important intellectual content.
- Research Article
- Abstract
- Introduction
- Material and methods
- Results
- Discussion
- Conclusion
- Compliance with ethical standards
- Ethics approval and consent to participate
- Consent for publication
- Availability of data and materials
- Competing Interests
- Funding information
- Authors’ contributions
- Acknowledgement
- References
Acknowledgement
This research was gratefully financed by grants of Ministry of Higher Education and Scientific Research and Unity of Bioactive and Natural Substances and Biotechnology UR17ES49, Faculty of Dental Medicine, University of Monastir.
- Research Article
- Abstract
- Introduction
- Material and methods
- Results
- Discussion
- Conclusion
- Compliance with ethical standards
- Ethics approval and consent to participate
- Consent for publication
- Availability of data and materials
- Competing Interests
- Funding information
- Authors’ contributions
- Acknowledgement
- References
References
- Ambudkar SV, Kimchi SC, Sauna ZE, Michael MG (2003) P-glycoprotein: from genomics to mechanism. Oncogene 22(47): 7468-7485.
- Sharom FJ (2008) ABC multidrug transporters: structure, function and role in chemoresistance. 9(1):105-127.
- Kruh GD (2003) Introduction to resistance to anticancer agents. Oncogene 22(47): 7262-7264.
- Lepper ER, Nooter K, Verweij J, Milin RA, William DF, et al. (2005) Mechanisms of resistance to anticancer drugs: the role of the polymorphic ABC transporters ABCB1 and ABCG2. Pharmacogenomics 6(2): 115-138.
- Labialle S, Gayet L, Marthinet E, Dominique R, Loris GB (2002) Transcriptional regulators of the human multidrug resistance 1 gene: recent views. Biochemical pharmacology 64(5-6): 943-948.
- Cui CB, Kakeya H, Osada H (1997) Novel mammalian cell cycle inhibitors, cyclotroprostatins A–D, produced by Aspergillus fumigatus, which inhibit mammalian cell cycle at G2/M phase. Tetrahedron 53(1): 59-72.
- De Souza PS, Da Cunha VF, Silva LFR, Raquel CM (2012) Cyclosporine A enables vincristine-induced apoptosis during reversal of multidrug resistance phenotype in chronic myeloid leukemia cells. Tumor Biology 33(4): 943-956.
- Lin Y, Shi R, Wang X, Han MS (2008) Luteolin, a flavonoid with potential for cancer prevention and therapy. Current cancer drug targets 8(7): 634-646.
- Chen Q, Liu S, Chen J, Qianqian Z, Shijie L, et al. (2012) Luteolin induces mitochondria-dependent apoptosis in human lung adenocarcinoma cell. Natural product communications 7(1): 29-32.
- Jeon YW, Suh YJ (2013) Synergistic apoptotic effect of celecoxib and luteolin on breast cancer cells. Oncology reports 29(2): 819-825.
- Tuorkey MJ (2016) Molecular targets of luteolin in cancer. European Journal of Cancer Prevention 25: 65.
- Rao PS, Satelli A, Moridani M, Marjorie J, Subrahmanyeswara RU (2012) Luteolin induces apoptosis in multidrug resistant cancer cells without affecting the drug transporter function: Involvement of cell line‐specific apoptotic mechanisms. International journal of cancer 130(11): 2703-2714.
- Luo X, Budihardjo I, Zou H, Slaughter C, Wang X (1998) Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94(4): 481-490.
- Yang JC, Lu MC, Lee CL, Guan YC, Yan YL, et al. (2011) Selective targeting of breast cancer cells through ROS-mediated mechanisms potentiates the lethality of paclitaxel by a novel diterpene, gelomulide K. Free Radical Biology and Medicine 51(3): 641-657.
- Trachootham D, Alexandre J, Huang P (2009) Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nature reviews Drug discovery 8(7): 579-591.
- Cheng AC, Huang TC, Lai CS, Min HP (2005) Induction of apoptosis by luteolin through cleavage of Bcl-2 family in human leukemia HL-60 cells. European journal of pharmacology 509(1): 1-10.
- Cho SG, Choi EJ (2002) Apoptotic signaling pathways: caspases and stress-activated protein kinases. BMB Reports 35(1): 24-27.
- Harker WG, Sikic BI (1985) Multidrug (pleiotropic) resistance in doxorubicin-selected variants of the human sarcoma cell line MES-SA. Cancer Research 45(9): 4091-4096.
- Qu XJ, Yang JL, Russell PJ, David G (2004) Changes in epidermal growth factor receptor expression in human bladder cancer cell lines following interferon-α The Journal of urology 172(2): 733-738.
- Debbasch C, Brignole F, Pisella PJ, Warnet JM, Ratet P, al. (2001) Quaternary ammoniums and other preservatives’ contribution in oxidative stress and apoptosis on Chang conjunctival cells. Investigative ophthalmology & visual science 42(3): 642-652.
- Singh NP, McCoy MT, Tice RR, Schneider EL (1988) A simple technique for quantitation of low levels of DNA damage in individual cells. Experimental cell research 175(1): 184-191.
- Collins AR, Dusinská M, Gedik CM, Rudolf Š (1996) Oxidative damage to DNA: do we have a reliable biomarker? Environmental Health Perspectives 104: 465-469.
- Miller DS, Dykes D, Polesky AH, Polesky HF, Justin M (1988) A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic acids research 16: 1215.
- Comte G, Daskiewicz JB, Bayet C, Conseil G, Viornery VA, et al. (2001) C-Isoprenylation of flavonoids enhances binding affinity toward P-glycoprotein and modulation of cancer cell chemoresistance. Journal of medicinal chemistry 44(5): 763-768.
- Youn MJ, Kim JK, Park SY, Yunha K, Channy P, et al. (2009) Potential anticancer properties of the water extract of Inontus obliquus by induction of apoptosis in melanoma B16-F10 cells. Journal of ethnopharmacology 121(2): 221-228.
- Ross JA, Kasum CM (2002) Dietary flavonoids: bioavailability, metabolic effects, and safety. 22: 19-34.
- Yang MY, Wang CJ, Chen NF, Wen HH, Fung JL, et al. (2014) Luteolin enhances paclitaxel-induced apoptosis in human breast cancer MDA-MB-231 cells by blocking STAT3. 213: 60-68.
- El Gueder D, Maatouk M, Kalboussi Z, Zaineb D, Hind C, et al. (2018) Heat processing effect of luteolin on anti-metastasis activity of human glioblastoma cells U87. 25(36): 36545-36554.
- Yin F, Giuliano AE, Van Herle AJ (1999) Growth inhibitory effects of flavonoids in human thyroid cancer cell lines. 9(4): 369-376.
- Chen Q, Liu S, Chen J, Qianqian Z, Shijie L, et al. (2012) Luteolin induces mitochondria-dependent apoptosis in human lung adenocarcinoma cell. 7(1): 29-32.
- Wang H, Luo Y, Qiao T, Zhaoxia W, Zhonghua H (2018) Luteolin sensitizes the antitumor effect of cisplatin in drug-resistant ovarian cancer via induction of apoptosis and inhibition of cell migration and invasion. 11: 93.
- Chen P, Zhang JY, Sha BB (2017) Luteolin inhibits cell proliferation and induces cell apoptosis via down-regulation of mitochondrial membrane potential in esophageal carcinoma cells EC1 and KYSE450 8: 27471.
- Yao X, Jiang W, Yu D, Zhaowei Y (2019) Luteolin inhibits proliferation and induces apoptosis of human melanoma cell in vivo and in vitro by suppressing MMP-2 and MMP-9 through PI3K/AKT pathway. Food & function.
- Rao PS, Satelli A, Moridani M, Marjorie J, Subrahmanyeswara RU (2012) Luteolin induces apoptosis in multidrug resistant cancer cells without affecting the drug transporter function: Involvement of cell line‐specific apoptotic mechanisms. 130(11): 2703-2714.
- Wang Q, Wang H, Jia Y, Hao P, Hui D (2017) Luteolin induces apoptosis by ROS/ER stress and mitochondrial dysfunction in gliomablastoma. 79(5): 1031-1041.
- Desagher S, Martinou JC (2000) Mitochondria as the central control point of apoptosis. 10(9): 369-377.
- Olichon A, Baricault L, Gas N, Emmanuelle G, Annie V, et al. (2003) Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome c release and apoptosis. 278(10): 7743-7746.
- Cheng AC, Huang TC, Lai CS, Min HP (2005) Induction of apoptosis by luteolin through cleavage of Bcl-2 family in human leukemia HL-60 cells. 509(1): 1-10.
- Horinaka M, Yoshida T, Shiraishi T, Susumu N, Miki W, al. (2005) Luteolin induces apoptosis via death receptor 5 upregulation in human malignant tumor cells. 24(48): 7180-7189.
- Xavier CP, Lima CF, Preto A, Raquel Seruca, Manuel FF, et al. (2009) Luteolin, quercetin and ursolic acid are potent inhibitors of proliferation and inducers of apoptosis in both KRAS and BRAF mutated human colorectal cancer cells. 281(2): 162-170.
- Lim W, Yang C, Bazer FW, Gwonhwa S (2016) Luteolin inhibits proliferation and induces apoptosis of human placental choriocarcinoma cells by blocking the PI3K/AKT pathway and regulating sterol regulatory element binding protein activity. 95(4): 82.
- Park SH, Ham S, Kwon TH, Man Sub K, Dong Hunet al. (2014) Luteolin induces cell cycle arrest and apoptosis through extrinsic and intrinsic signaling pathways in MCF-7 breast cancer cells. 33(2): 219-231.
- Su Bog Y, Jung HL, Hae Young C, Kwang SI, Song JB, e et al. (2003) Inhibitory effects of luteolin isolated fromixeris sonchifolia hance on the proliferation of hepg2 human hepatocellular carcinoma cells 26(2): 151-156.
- Wu B, Zhang Q, Shen W, Jun Z (2008) Anti-proliferative and chemosensitizing effects of luteolin on human gastric cancer AGS cell line. 313(1-2): 125-132.
- Almeida P, Boleti AP, Rudiger A, ValdirVeiga-JuniorMarneVasconcellos, et al. (2014) Anticancer Effects of Triterpenes from Protium Paniculatum in the Multidrug-Resistant Human Uterine Sarcoma MES-SA/Dx5. Free Radical Biology and Medicine: S120.






























