Primary Adenoid Cystic Carcinoma of the Breast with Dcis- Radiological Review of an Extreme Rarity
Kameswari Padmanabhan*, Sudhakar S, Avinash Kesari, Mahesh Kumar, Lohith G, Indresh Desai, Yashaswini K, Shivakumar Swamy, Veena Ramaswamy and Kumar Kallur
Fellow in Breast Imaging, HCG Enterprises, Bangalore , India
Submission: April 15, 2021; Published: May 11, 2021
*Corresponding Address: Kameswari Padmanabhan, Fellow in Breast Imaging, HCG Enterprises, Bangalore , India
How to cite this article: Kameswari P, Sudhakar S, Avinash K, Mahesh K, Lohith G, et al. Primary Adenoid Cystic Carcinoma of the Breast with Dcis- Radiological Review of an Extreme Rarity. Canc Therapy & Oncol Int J. 2021; 18(4): 555995 .DOI: 10.19080/CTOIJ.2021.18.555995
Abstract
Primary Adenoid cystic carcinoma (ACC) of the breast is a rare tumor, known to occur in only 0.1% of all breast cancers and even rarer are presentations with another tumor sub-type. ACC is known to have a good prognosis and it is, therefore, important to make an accurate diagnosis and provide timely management. We present a case of an elderly woman with a lump in her right breast. Digital mammogram revealed an ill-defined mass like lesion with pleomorphic calcifications, and ultrasound showed an ill-defined hypo-echoic lesion with internal calcifications. Core needle biopsy from the lesion revealed an adenoid cystic carcinoma. Staging PET-CT showed the lesion to be metabolically active. Subsequently, the patient underwent a right lumpectomy and sentinel node excision. Histopathology examination revealed tumor histology of adenoid cystic carcinoma, cribriform type with presence of DCIS, and free sentinel lymph nodes with ‘triple negativity’. She underwent Radiotherapy and follow-up PET CT at 8 months revealed no recurrent mass.
Keywords: Salivary glands; Digital Mammography; Pleomorphic calcifications; Echogenicity
Introduction
Adenoid cystic carcinoma is a rare tumor of the breast occurring in <0.1 % of cases. It is known to occur more commonly in the salivary glands, bronchi, endocervix, esophagus, lungs, and the skin [1,2]. Adenoid Cystic Carcinoma of the breast shares histopathological features with its salivary gland variant and is characterized by the dual presence of both epithelial and myo-epithelial cells [3,4]. When occurring in the breast, it has been known to have a favorable prognosis with rare incidence of metastasis or recurrence. Due to limited literature on this tumor, not much is known about the presentation and imaging of this subtype of breast carcinoma. And there are even fewer studies on ACCs combined with DCIS. Our case report strives to add to the present literature of imaging features of this rare tumor with a DCIS component along with a literature review.
Case Report
A 67-year-old woman presented to our hospital with history of a palpable lump in the right breast which she had noticed over the previous 25 days. She gave no history of pain, nipple discharge, or retraction. There was no family history of carcinoma breast, and her surgical history included a fibro-adenoma excision from the left breast 3 years ago. The patient had her first child at the age of 21 and presently has three children and she attained menopause at the age of 49. On clinical examination, a 2.5 × 2.5cm well-defined mass that was not freely mobile was identified in the right breast in the retro-areolar position. One lymph node was palpable in the right axilla and nipple discharge was not elicited.
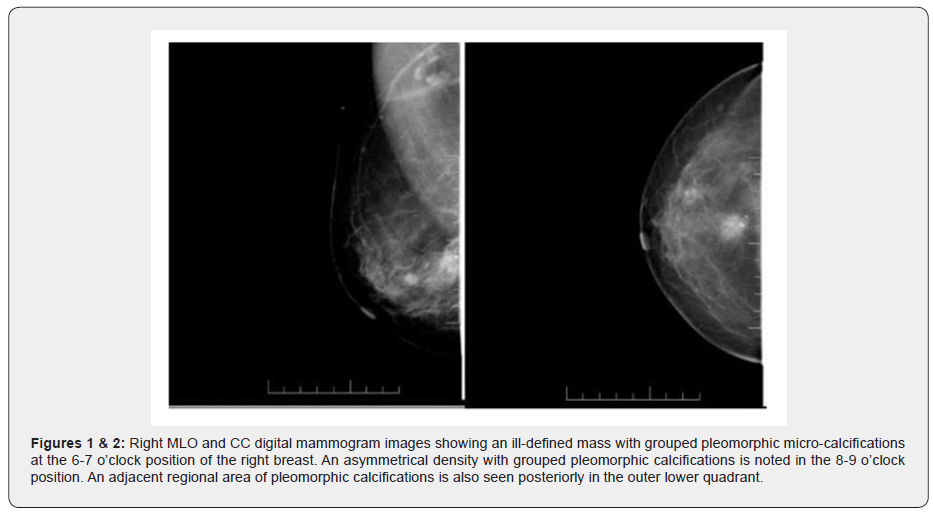
Digital Mammography of both her breasts was done which revealed an ill-defined nodular soft tissue density lesion with grouped pleomorphic micro-calcifications at the 6-7 o’clock position in the right breast. Another asymmetrical density with pleomorphic calcifications was noted at the 8-9 o’clock position as well as another area adjacent regional area of pleomorphic calcifications in the outer lower quadrant of right breast.
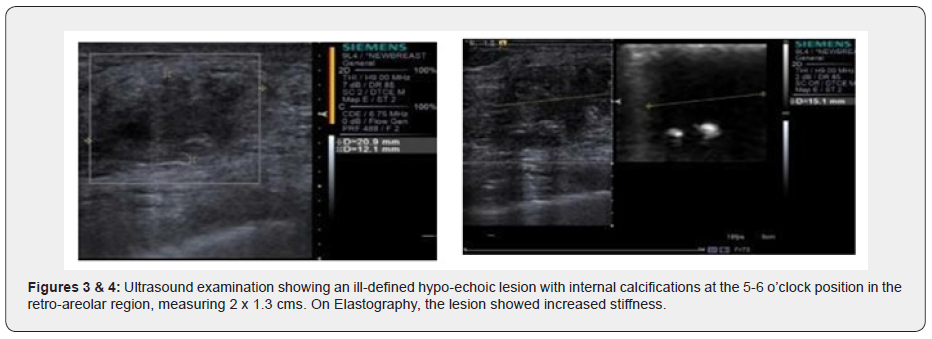
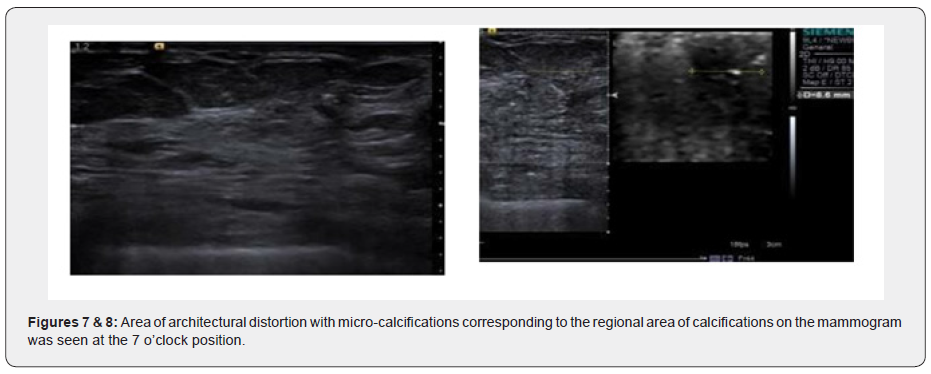
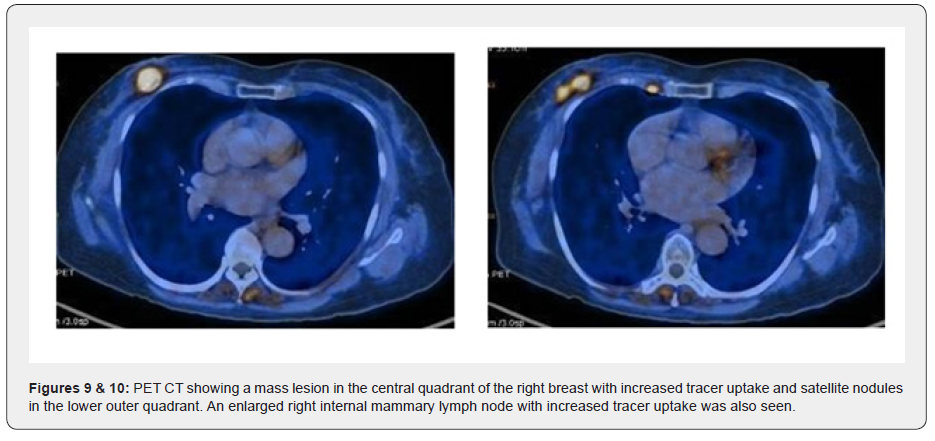
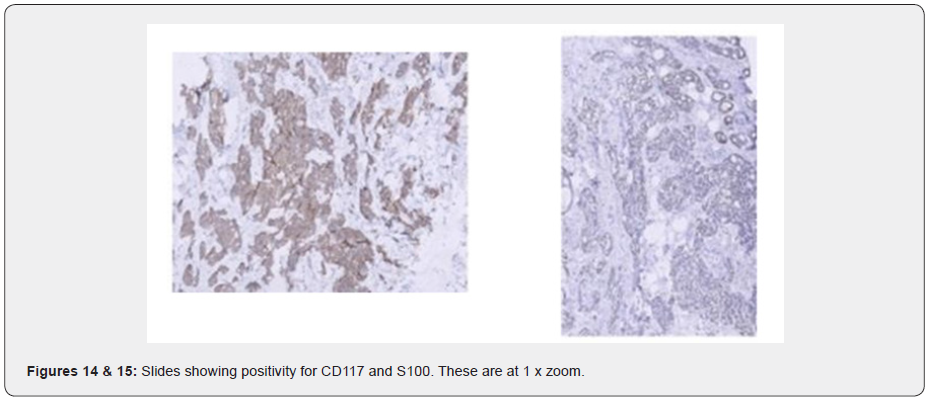
Ultrasound examination showed an ill-defined hypo-echoic lesion with internal calcifications at the 6 o'clock position in the retro-areolar region. Another partially circumscribed hypo-echoic lesion with internal calcifications corresponding to the asymmetrical density on Digital mammogram was seen at the 9 o’clock position. Both lesions showed minimal vascularity. An adjacent group of pleomorphic calcifications was also noted at the 7 o’clock position which corresponded to the regional area of calcifications on the Mammogram. Ultrasound done over the axilla showed prominent sub-centimeter lymph nodes along with cortical thickening, however no abnormal change in echogenicity or vascularity was noted. A BIRADS V classification was given (highly suspicious for malignancy) and USG was followed with a core needle biopsy using a 16-gauge automated biopsy gun. Histopathology revealed morphology and immunohistochemistry features of ‘Triple negative’ Adenoid Cystic Carcinoma with CD117, CK7 and S100 positivity. The patient then underwent staging PET CT as part of our institution’s protocol which showed a metabolically active mass lesion in the central quadrant of the right breast with other metabolically active satellite nodules (SUV- 15.2) in the lower outer quadrant and an enlarged active right internal mammary lymph node (SUV-7.8).
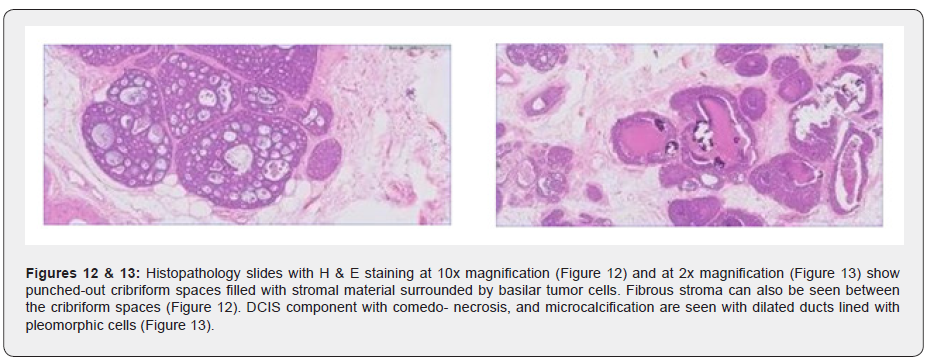
The patient underwent wide local excision with right Sentinel lymph node biopsy. The cut surface of the lesions appeared grey and ill-defined. Subsequently HPE revealed multifocal Adenoid cystic carcinoma of the cribriform type, negative for ER/ PR and HER/Neu2, Grade IIi nuclear grade (high) with the presence of DCIS and no lymph node metastasis or vascular invasion and was characterized as Pathologic stage -(m) PT1cN0(sn). Radiotherapy was administered in 15 fractions of 40 Gy in Phase 1 and a tumor bed boost was given in phase 2 which was 10 Gy over 4 fractions. Follow-up PET CT eight months later revealed no local recurrence or distant metastases (Figures 1-15).

Discussion
Primary Adenoid Cystic Carcinoma is a rare tumor of the breast, accounting for less than 0.1% of the cases, and is commonly seen in the salivary glands and bronchi. Unlike the salivary gland counterpart ACC of the breast has been found to have a favorable prognosis with low incidence of recurrence. Characterized initially as a 'Cylindroma’ in 1856 by Billroth and later described by Geschickter and Copeland as ‘adenocystic basal cell cancer of the breast’ in 1945, it is usually found in the fifth and sixth decades of life in women, although the age range can be from the second to the ninth decade with few cases being reported in men and children [5,6]. There is no specific predilection for this tumor to develop bilaterally, although another carcinoma may develop in the contralateral breast or in the same breast [6]. The most common clinical presentation is that of a palpable breast mass. Around 50% of cases show a predilection to the sub-areolar region and the surrounding area with other areas commonly affected being the upper quadrants [1,3,7]. They may also have pain and tenderness with nipple retraction. Rarely the mass shows fixation to the underlying muscles, nipple, or skin [7,8]. These tumors are slow growing over the course of months and years with instances where masses have been found incidentally on screening mammograms [9].
This type of Breast cancer shows varying imaging appearances. On mammography, they can be found as irregular high-density masses with lobulations, indistinct margins, spiculations, or asymmetrical densities [2,4]. The presence of microcalcifications is rare although few cases have been reported [6]. Our case also had internal pleomorphic calcifications which has only been reported in few cases [6]. They may also have benign imaging features, making them difficult to distinguish from fibroadenomas [6,10]. In some cases, they may remain occult in a mammogram owing to the presence of dense breast tissue [2]. On ultrasound imaging, the lesion may appear as predominantly hypo-echoic or in some cases heterogeneous. The internal echo of the mass can also present as mixed cystic and solid as was seen in the case report by Wangli et al. [5]. And it also usually presents as a irregularly shaped lesion with angular, micro- lobulated, or unclear margins [2,5,11]. Doppler imaging usually reveals minimal vascularity [10], which was observed in our case.
MRI imaging of Adenoid Cystic Carcinoma remains controversial, and information is limited. Glazebrook et al. described variable MR imaging appearances with lesions being either lobulated or irregular with irregular or spiculated margins. Enhancement was found to be rapid and heterogeneous on contrast administration with a combination of persistent, plateau, and washout kinetics. On T2 imaging, larger lesions and those which were the solid variant had a high signal, while the smaller lesions showed iso-intensity to surrounding breast tissue [2]. In a study by Tsuboi et al. ACC showed a well-circumscribed round lesion with rapid and marked enhancement (extending towards the center from the periphery) in which there was no washout of the contrast agent [2,12]. In another study, Gorane Santamaria et.al described the MRI features of a case of adenoid cystic carcinoma of the glandular (cribriform) type which showed moderately hyperintense signal on T2-weighted turbo SE images, surmising that was due to the myxoid stroma and cribriform pattern of the tumor [12]. In a report by Kasawaga et al. MRI showed strong peripheral enhancement of one tumor, while in the other early enhancement was noted [13]. Okamoto et al. also described some masses showing benign delayed enhancement [7].
Histopathological examination usually reveals a triple-negative tumor with a biphasic cellular pattern with epithelial/luminal and myoepithelial/abluminal cells [14]. This subtype comes under the rare and salivary gland tumors in the revised WHO classification and is sub-categorized as
a) classic adenoid cystic carcinoma
b) Solid basaloid adenoid cystic carcinoma
c) Adenoid cystic carcinoma with high grade transformation [15].
The biphasic cell type can be arranged in tubular-trabecular, cribriform, or solid-basaloid patterns. These structures are lined by the luminal and myo-epithelial cells which are the pseudo-lamina and true glandular spaces. The luminal cells surround true gland lumina and the myoepithelial-like cells line the cribriform spaces. These myoepithelial-like cells sometimes contain basement membrane like material.
ACC of the breast is usually negative for ER, PR, and HER [1], although ER and PR positive cases have been reported. ER positivity may point towards a non -pure ACC or invasive cribriform carcinoma. Immunohistochemically, the luminal cells ACC is CD117 positive, helping it to distinguish from other cancers [5]. These cells are also positive for CK7, CK8/18, Epithelial membrane antigen. The myoepithelial basal cells show reactivity for vimentin, myo-epithelial markers like S-100 protein, calponin, actin, p63, basal cytokeratins like CK5/6, CK14, and EGFR (epidermal growth factor receptor which could be a therapeutic agent in the future [3]. In our case, the luminal cells were positive for CD117 and CK7 and the myo-epithelial basal cells showed reactivity for S100, aiding our diagnosis of ACC, although they were negative for p63. ACC is also divided into three grades based on the proportion of solid growth in the tumor- with grade I having no solid elements, grade II having less than 30%, and Grade II having more than 30% solid component4. In our case there was no pathological evidence of Solid components making it Grade I. In addition, Kleer et al. [16] graded the tumor cell nuclei from nuclear grade 1 to 34 and in our case was found to be II and categorized as high. And unlike other triple-negative tumors, proliferative activity is found to be low in ACC and is diagnosed with proliferative markers like Ki-67. In our case, KI-67 was found to be 30% suggesting a 'high' Ki-67 expression [17].
Additionally, our case had a DCIS component, a rare phenomenon, with extremely limited literature and publications on this entity. Our research found one case report by Burusapat et al. [11] who presented a case that showed an irregular mass in the sub-areolar region without the presence of microcalcifications. The initial core biopsy from the lesion and the lymph node showed triple-negative Invasive ductal carcinoma with nodal metastasis. However, the final HPE result after surgery revealed an ACC with DCIS component of the high-grade type with microcalcifications. Although we found another case report by Kontos et al. [18] it was a case of ACC with an infiltrating ductal component. In yet another case report by Righi et al. [19] the tumor had both ACC and IDC components, and the ACC component comprised 70% of the tumor. Additionally, the DCIS-IDC part showed alterations in connection to the ACC component hinting that the part went through a transformation and developed into a more aggressive tumor. It is quite clear, that a case of ACC along with DCIS, except for one case report by Burusapat et al. [11] and another with a combination of DCIS and IDC with ACC, has never been reported, as far as our research has shown.
The differential diagnosis includes invasive cribriform/tubular carcinoma, cribriform Invasive ductal carcinoma, and DCIS and due to its histological similarity to Invasive ductal carcinoma, biopsy specimens can be wrongly diagnosed. A way of differentiating between cribriform carcinoma and ACC is that cribriform carcinoma does not contain basement membrane material and expresses ER and PR. Cribriform Carcinomas are also negative for CD117. A benign condition that should be included in the differential is collagen spherulosis. The main distinguishing feature between them is the formation of ductal epithelium in Collagen spherulosis. Ductal lumina formation in ACC is rare and if they occur, are smaller [3,11,20].
Treatment and management – Staging is done before treatment is started, which is based on AJCC staging criteria. In our case, the clinical and anatomic staging would be T1cN0M0 and Stage IA. The pathologic prognostic stage is based on all clinical information, biomarker information, and information from surgery and resected tissue. This is done for patients who undergo surgery first before any adjuvant radiotherapy or chemotherapy [21]. Here, the final pathologic stage we found was (m) pT1cN0(sn). Coming to management, there are no definite protocols on treatment guidelines. Patients with local disease may undergo breast conservation surgery while those with more invasive disease benefit from a mastectomy. Adjuvant radiotherapy may play a role to avoid positive tumor margins after BCT, as recurrences have been reported. In the study by glazebrook et al. one of the patients who underwent lumpectomy had multiple recurrences but no evidence of distant metastasis, so an addition of radiotherapy for patients undergoing lumpectomy may prove beneficial [2]. Since lymph node metastasis is rare in cases of ACC, axillary lymph node dissection may not always be needed [20]. And with newer avenues opening in the treatment of cancer, such as the use of targeted therapies like MYBNFIB fusion gene, the treatment, and management of ACC may transform further [3]. Not much information exists on the benefit of hormonal therapy and is administered in cases where there is hormone-positive status as is outlined in the case report by Fatma et al. [22]. And in cases of triple-negative status, adjuvant chemotherapy may also be regarded in treatment [1]. Even though distant metastasis is rare, patients must be kept on long-term follow-up since metastases can occur without lymph node involvement. Usual sites of metastasis are the bone, liver, kidney and are like the sites of metastasis in ACC of the salivary glands [22].
Conclusion
Adenoid cystic carcinoma is a rare tumor of the breast but if diagnosed early, has a favorable prognosis in prolonging patient life. It has specific clinicopathologic tumor patterns which help in distinguishing it from other triple-negative variants. And even though, it occurs usually as a 'triple negative' cancer, it is a low-grade tumor with an indolent growth pattern and rarely metastasizes and for this reason, should be included in the initial workup of the patient. Care in the histochemical analysis should be taken since we have seen that ACC, although a low-grade tumor can occur with other tumor subtypes, possibly making it aggressive, and should be kept in mind in the follow-up of the patient.
References
- Kashiwagi S, Asano Y, Ishihara S, Morisaki T, Takashima T, et al. (2019) Adenoid Cystic Carcinoma of the Breast: A Case Report. Case Rep Oncol 12(3): 698-703.
- Katrina NG, Carol R, Robin L, Edgardo IG, Judy CB (2010) Adenoid Cystic Carcinoma of the Breast. American Journal of Roentgenology 194(5): 1391-1396.
- Bhutani N, Kajal P, Singla S (2018) Adenoid Cystic Carcinoma of the Breast: Experience at a tertiary care centre of Northern India. International Journal of Surgery Case Reports 51: 204-209.
- Naeem M, Maria Z, David HB, Laura B, Guihua C, et al. (2020) The unusual suspects-Mammographic, sonographic, and histopathologic appearance of atypical breast masses. Clin Imaging 66: 111-120.
- Wang H, Liu F, Gu R, Li Y, Su F (2017) Rare imaging appearance of adenoid cystic carcinoma of the breast: A case report. Mol Clin Oncol 7(3): 473-475.
- Boujelbene N, Khabir A, Boujelbene N, Jeanneret SW, Mirimanoff RO, et al. (2012) Clinical review--breast adenoid cystic carcinoma. Breast 21(2): 124-127.
- Okamoto Y, Sumiyama Y, Arima Y, Sakuta M, Okuda T, et al. (2001) A case of adenoid cystic carcinoma (ACC) of the breast and review of the utility of preoperative imaging diagnose. Breast Cancer 8(1): 84-89.
- Santamaría G, Velasco M, Zanón G, Farrús B, Molina R, et al. (1998) Adenoid cystic carcinoma of the breast: mammographic appearance and pathologic correlation. AJR Am J Roentgenol 171(6): 1679-1683.
- Tummidi S, Shubhra P, Deepti J, Ashwani T, Anjaly M, et al. (2020) Adenoid Cystic Carcinoma Breast: a Rare Entity. Indian J Surg Oncol 11: 226-231.
- Tang W, Wei JP, Ya JG, Hui Z, Ting TJ, et al. (2015) Imaging Manifestation of Adenoid Cystic Carcinoma of the Breast. J Comput Assist Tomogr 39(4): 523-530.
- Burusapat C, Naphan B, Kittisak W, Pongsit C, Parinya P, et al. (2020) Mammary adenoid cystic carcinoma presenting with Ductal carcinoma in situ and axillary lymph node metastasis. Journal of Surgical Case Reports.
- Santamaría G, Martín V, Xavier B, Xavier C, Blanca F, et al. (2010) Radiologic and pathologic findings in breast tumors with high signal intensity on T2-weighted MR images. Radiographics 30(2): 533-548.
- Kasagawa T, Suzuki M, Doki T, Fujimori T, Itami M, et al. (2006) Two cases of adenoid cystic carcinoma: preoperative cytological findings were useful in determining treatment strategy. Breast Cancer 13(1): 112-116.
- Senger JL, Kanthan R (2016) Adenoid Cystic Carcinoma of the Breast A Focused Review. JSM Surg Oncol Res 1(2): 1008.
- Agarwal I, Blanco L (2021) WHO classification. PathologyOutlines.com website.
- Kim M, Dae WL, Jin I, Koung JS, Bhumsuk K, et al. (2014) Adenoid cystic carcinoma of the breast: a case series of six patients and literature review. Cancer Res Treat 46(1): 93-97.
- Soliman NA, Yussif SM (2016) Ki-67 as a prognostic marker according to breast cancer molecular subtype. Cancer biology & medicine 13(4): 496-504.
- Kontos M, Karles D, Petrou A, Paraskevi THA (2011) Adenoid cystic carcinoma intermingled with ductal carcinoma of the breast: a case report and review of the literature. J Med Case Reports 5: 437.
- Righi A, Manuela L, Luca M, Federica F, Dario DB, et al. (2011) Adenoid Cystic Carcinoma of the Breast Associated with Invasive Duct Carcinoma: A Case Report. International Journal of Surgical Pathology 19(2): 230-234.
- Thomas DN, Asarian A, Xiao P (2019) Adenoid cystic carcinoma of the breast. Journal of Surgical Case Reports.
- Kalli S, Semine A, Cohen S, Naber SP, Makim SS, et al. American Joint Committee on Cancer's Staging System for Breast Cancer, Eighth Edition: What the Radiologist Needs to Know. Radiographics: a review publication of the Radiological Society of North America Inc 38(7): 1921-1933.
- Dhouib F, Mouna K, Wafa M, Nejla F, Wicem S, et al. (2020) Adenoid cystic carcinoma of the breast. PAMJ Clinical Medicine 3(130).






























