Leptomeningeal Involvement in Lyme’s Disease: A Rare Case of Lymphocytic Meningitis of Lyme’s disease Mimicking Metastatic Leptomeningeal Carcinomatosis: A Case Study
Zebi Fatima*, Soumya Pulipati, Babar Shahzad and Adedayo Onitilo
Marshfield Clinic Health System, Marshfield, Wisconsin, USA
Submission: April 30, 2021; Published:May 10, 2021
*Corresponding Address: Zebi Fatima, Marshfield Clinic Health System, Marshfield, Wisconsin, 1000 N Oak Ave, Marshfield 54449, USA
How to cite this article: Zebi F, Soumya P, Babar S, Adedayo O. Leptomeningeal Involvement in Lyme’s Disease: A Rare Case of Lymphocytic Meningitis of Lyme’s disease Mimicking Metastatic Leptomeningeal Carcinomatosis: A Case Study. Canc Therapy & Oncol Int J. 2021; 18(4): 555994. DOI: 10.19080/CTOIJ.2021.18.555994
Abstract
We present a case of a 54-year-old female who was diagnosed with infiltrating ductal carcinoma and was in complete pathological remission post partial mastectomy and neoadjuvant chemotherapy for 11 years. In 2020, the patient presented with a throbbing headache followed by Bell’s palsy. An MRI of the brain and spinal cord was performed which showed a small focus of enhancement in the right superior parietal lobule with additional sulcal enhancement in the left parietal lobe which was concerning for the intracranial metastatic process questioning the leptomeningeal component. Since the patient had a history of breast cancer, prompt follow-up with labs, CT of the chest, spinal fluid analysis was performed. The patient was found to have an abnormal Lyme serology with a positive IgM antibody. Breast cancer antigen 15–3 was within the normal range. The patient received treatment for Lyme’s disease and showed improvement in her symptoms.
Keywords: Leptomeningeal spread; Lyme’s; Carcinomatosis; CSF analysis; Metastasis
Introduction
Leptomeningeal spread is a serious complication of advanced malignancy. The most common cancers causing leptomeningeal metastasis include gastrointestinal malignancies, lung cancers, breast cancer, and melanoma [1]. Clinical symptoms might include nausea/vomiting, varying degrees of headache, cognitive dysfunction, seizures, radicular pain, cranial nerve dysfunction, cerebellar signs [2]. MRI brain and spine reveals contrast enhancement of leptomeninges, dural or cranial nerves. Cerebrospinal fluid analysis is usually performed after MRI and usually shows high opening pressure, elevated protein, low glucose, lymphocytic pleocytosis, and cytology being positive for malignant cells. CSF cytology is the diagnostic cornerstone of neoplastic meningitis due to the high rate of specificity [3]. Although, infectious etiologies always need to be ruled out before committing to a diagnosis of leptomeningeal carcinomatosis as many CNS infections can present as similar MRI findings. We are presenting a case report of a patient with a pre-existing diagnosis of breast cancer in the past who presented with headaches and Bell’s palsy and showed leptomeningeal enhancement on MRI and was later found to have Lyme’s disease which responded to treatment.
Case Report
A 54-year-old right-handed female presented to her primary care physician with a complaint of throbbing headaches for the last few days. Due to her remote history of breast cancer (diagnosed 11 years ago in 2009), an MRI with contrast was requested. 5 days later she presented with facial droop and difficulty closing her right eye. She also reported a skin rash 1 week ago initially occurring on the right breast and her abdomen and later extending to the extremities. The rash was large and erythematous without itching. She presented to ER and was diagnosed with right-sided peripheral facial palsy. Lyme serology was done which came back abnormal with positive IgM antibody and negative IgG antibody. Her comprehensive metabolic panel was normal except for elevated alkaline phosphatase at 137. CBC was normal. Breast cancer antigen 15–3 was within normal limits. ANA was performed which was low positive at 1 is 218 and ESR was normal. Her lipid panel which was done 1 month ago showed elevated cholesterol at 234, LDL at 150, triglycerides at 229, normal non-HDL C, and low HDL at 38. Her brain MRI showed a small focus of enhancement in the right superior parietal lobule with additional sulcal enhancement in the left parietal lobe which was concerning for intracranial metastatic process questioning leptomeningeal component in the given clinical setting but also chronic microvascular ischemia and involutional changes.
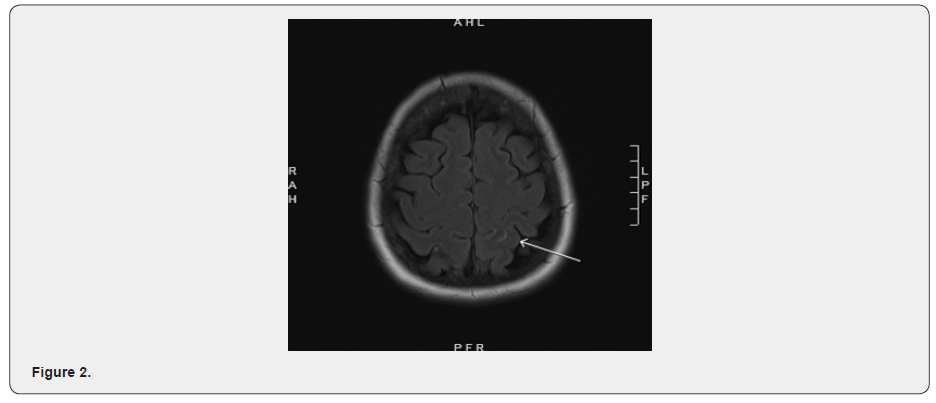
MRI brain axial T2 FLAIR post (June 2020) showed a punctate focus of enhancement in the surface of the right superior parietal lobule (Figure 1). Also noted, subtle sulcal hyperintensity and enhancement present in the left superior parietal lobule (Figure 2) and additional smooth dural-based contrast enhancement along the falx cerebri. While the latter might be vascular due to venous contrast bleeding, the parietal enhancing focus was concerning for metastatic deposit with a probable leptomeningeal component. No significant surrounding parenchymal edema or mass-effect was noted. Important breast cancer history includes infiltrating ductal carcinoma in the right breast, which was diagnosed in 2009, clinical-stage IIa (cT2 cN0 cM0), 2.4 x 2.0 x 2.3 cm on ultrasound, grade 3 with intraductal carcinoma, ER 3+, PR 2+, HER-2/neu 2+, FISH unamplified (ratio 1.03). MRI of the breast showed a right breast mass 2.8 x 3.2 cm with a 1.6 cm linear extension from the main mass. An additional 0.6 cm lesion was found in the left breast. The patient underwent a right breast ultrasound-guided biopsy which confirmed the diagnosis. She received dose-dense adjuvant chemotherapy 4 cycles which were completed in the next 2 months following the diagnosis. She also received weekly paclitaxel 80 mg/m² for 12 weeks. This was followed by right partial mastectomy and sentinel lymph node sampling. The patient also received right whole breast irradiation. The above treatment was followed by tamoxifen 20 mg orally from 2010 to 2015.

Important family history of cancer included a history of breast cancer in mother and sister. There was also a history of uterine cancer in the sister. Maternal grandfather had a history of stomach cancer. History of lung cancer was positive in maternal uncle, paternal uncle, paternal aunt. Maternal uncle was diagnosed with leukemia. There was a history of throat cancer in another maternal uncle. Maternal aunt had a history of bone cancer.
A CT scan of the chest with contrast was performed which did not show any definitive metastatic disease changes. A CT of the abdomen with contrast on the same day showed a hypodense lesion in the liver which was less prominent than in 2009 at the time of her breast cancer diagnosis and was consistent with a hemangioma. There was also a small amount of ascites seen in the pelvis of questionable etiology and significance. The patient underwent a breast mammogram which was described as benign. Her spinal fluid analysis was carried out and showed normal cell count, protein and glucose. Lyme’s PCR in the spinal fluid was negative. Cytology showed rare, atypical, degenerated cells.
After the serology for Lyme’s disease was found to be positive, the patient was treated with doxycycline for 3 weeks. Followup after 3 weeks showed improvement in symptoms including headache and facial palsy. Considering no evidence of ongoing disease as seen on recent imaging studies elsewhere, a followup brain MRI study with contrast was scheduled after 2 months. Since her ESR and ANA were essentially within normal range, additional testing was deferred. On a follow-up visit with oncology, the patient was found to have an improvement in her right-sided facial palsy and headaches. No further oncology workup was recommended.
Discussion
Leptomeningeal metastasis (neoplastic meningitis) is a rare but devastating complication of advanced stages of cancer. Since multiple areas of the craniospinal axis are involved, patient may present with a broad range of signs and symptoms. Diagnosis is confirmed by neuroimaging and CSF analysis. It is diagnosed in approximately 5% of patients with metastatic cancer, but asymptomatic or undiagnosed involvement is more common. Autopsy findings may reveal an average of 20% cases of metastatic cancer and are much higher in some tumor types. In 50 to 80% of patients, coexisting brain metastasis can also be present. Breast cancer is the most common solid tumor giving rise to leptomeningeal carcinomatosis with an average of 12 to 35% as compared to lung cancer averaging 10 to 26% of solid tumors, melanoma 5 to 25%, GI malignancies 4 to 14%, and cancers of unknown primary 1 to 7% [4]. According to the literature, the median time for the leptomeningeal spread in breast cancer cases is 18 months. But there have been case reports of a long period between the initial diagnosis and appearance of leptomeningeal spread if 144 months [5]. Multifocal neurological signs and symptoms are the hallmarks of the presentation of leptomeningeal carcinomatosis. The most common presenting symptoms include headache, nausea and vomiting, leg weakness, ataxia, altered mental status, diplopia, and facial weakness. Seizures can occur in up to one-fourth of the patients.
Contrast-enhanced MRI of the brain and spine should be performed in all patients who have suspected disease before doing a lumbar puncture. Findings on MRI that suggested diagnosis include nodular and linear leptomeningeal enhancement, thickening, and enhancement of cranial nerves or nerve roots, including the cauda equina along with hydrocephalus. A typical MRI in the appropriate clinical setting is sufficient for establishing the diagnosis without lumbar puncture. But in cases such as those mentioned in the case report, patients might present as a diagnostic dilemma with a completely different disease presenting similarly to leptomeningeal carcinomatosis. In which case CSF analysis becomes a test of high importance. Malignant cells identified in the CSF establish the diagnosis of leptomeningeal carcinomatosis. However, cytology can be negative, and up to 20% of the patients. CSF flow cytometry may be particularly helpful in diagnosing leptomeningeal carcinomatosis from hematological malignancies but is not routinely indicated for evaluation of solid tumors. Elevated tumor marker concentrations in CSF might be helpful in a selected few patient but is not routinely performed except in cases where there is high clinical suspicion and multiple cytologic samples are negative.
Central nervous system involvement in Lyme’s disease can present in several forms. Lymphocytic meningitis remains the most common. Rarely, inflammation of the brain and/or spinal cord parenchyma (encephalomyelitis) can occur. Clinically, lymphocytic meningitis of Lyme’s disease is hard to distinguish from viral meningitis with headache, fever, photosensitivity, and neck stiffness. Among all the Lyme disease cases verified by CDC, meningitis in isolation was present in approximately 2% of the patients. Cranial neuropathies associated with Lyme’s usually occurs early in infection and are abrupt in onset. It can involve any of the cranial nerves but the seventh (facial) is by far the most common occurring in 8% of Centers for Disease Control and Prevention (CDC) confirmed cases of Lyme’s disease [6] as seen in our patient. Most patients have elevated concentrations of Lyme’s antibody and peripheral blood with a prominent IgM component. Sometimes, cranial neuropathies occur even before the patient has become seropositive, in which case a follow-up titer in several weeks is typically diagnostic. Other nerves involved include the nerves innervating extraocular muscles, the vestibulocochlear nerve, trigeminal nerve, and infrequently the lower cranial nerves. Very few cases of a breast cancer patient with proven Lyme’s disease have been reported in the literature. These cases along with the case presented above expressed the importance of using both MRI and CSF analysis to differentiate Leptomeningeal Carcinomatosis with CNS Lyme’s disease. Although MRI has a high sensitivity for the findings of leptomeningeal carcinomatosis, similar findings can be present in meningitis associated with Lyme’s disease. In the case presented above, the patient showed complete resolution of symptoms after treatment of Lyme’s disease. Lumbar puncture with CSF analysis showed be performed to confirm the diagnosis of leptomeningeal metastasis before initiating treatment for suspected cancer recurrence.
Conflict of Interest
None.
References
- Fernando Matos, Luis Cerqueira (2019) Dural and Leptomeningeal Spine Metastases of Breast Cancer. Case Rep Radiol 2019: 4289362.
- Stefanie Fischer, Johannes Weber, Isabelle Senn-Schonenberger, Thomas Cerny, Thomas Hundsberger, et al. (2014) Neuroborreliosis Mimicking Leptomeningeal Carcinomatosis in a Patient with Breast Cancer. J Investig Med High Impact Case Rep 2(1): 2324709614529417.
- Georgios Rigakos, Chrysoula I Liakou, Naillid Felipe, Dennis Orkoulas-Razis, Evangelia Razis (2018) Clinical Presentation, Diagnosis, and Radiological Findings of Neoplastic Meningitis. Cancer Control 24(1).
- Kaplan JG, DeSouza TG, Farkash A, B Shafran, D Pack, et al. (1990) Leptomeningeal metastases: comparison of clinical features and laboratory data of solid tumors, lymphomas and leukemias. J Neurooncol 9(3): 225-229.
- Lena Bonig, Nora Mohn, Jonas Ahlbrecht, Ulrich Wurster, Peter Raab, et al. (2019) Leptomeningeal Metastasis: The Role of Cerebrospinal Fluid Diagnostics. Front Neurol 10: 839.
- Centers for Disease Control and Prevention (CDC) (2007) Lyme disease--United States, 2003-2005. MMWR Morb Mortal Wkly Rep 56(23): 573-576.






























