Role of Glucose on Breast Cancer Patients
Ishita Saha1 and Rabindra Nath Das2*
1Department of Physiology, Calcutta Medical College and Hospital, India
2Department of Statistics, The University of Burdwan, India
Submission:October 15, 2020; Published:December 14, 2020
*Corresponding Address:Rabindra Nath Das, Department of Statistics, The University of Burdwan, Burdwan, West Bengal, India
How to cite this article:Ishita S, Rabindra N D. Role of Glucose on Breast Cancer Patients. Canc Therapy & Oncol Int J. 2020; 17(4): 555967.DOI:10.19080/CTOIJ.2020.17.555967
Editorial
Cancer and diabetes mellitus (DM) are frequently occurring diseases with frightening impact on human health worldwide. In epidemiological studies, it is observed that people with DM are always at higher risk for several types of cancer [1-4]. Some research reports have pointed that there is a direct link between overweight and cruelty of breast cancer (BC) [5-7]. Correlation between the BC prognosis and metabolic syndrome has been reported in [8]. The following inquiries are surveyed in the present editorial report.
i.Are there any effects of glucose with BC patients and BC markers?
ii.If it is affirmative, what are the relations of glucose with BC patients and BC markers?
iii.What are the effects of glucose on BC patients and BC markers?
The above queries are searched in the editorial note based on a real data set of 116 normal and BC women along with 10 study variables, and the data set is available in the UCI Machine Learning Repository, and it is clearly illustrated in [9,10]. For immediate applications, the 10 study variables are presented as follows.
a.Age (years),
b.BMI (kg/m2),
c.Homeostasis model assessment score insulin resistance (HOMA-IR),
d.Insulin (μU/mL),
e.Glucose (mg/ dL),
f.Resistin (ng/mL),
g.Adiponectin (μg /mL),
h.Monocyte chemoattractant protein-1 (MCP-1) (pg/dL),
i.Leptin (ng/mL),
j.Patient type (PT) (1=Healthy controls; 2= BC patients).
The above inquiries can only be surveyed by modeling glucose on the rest factors. Note that glucose is a positive continuous heteroscedastic random variable that can be modeled by joint generalized linear models (JGLMs) under both the lognormal and gamma distributions [11,12]. Joint gamma glucose model fit gives better results than lognormal fit, so only the gamma model fit outcomes are displayed in Table 1. In addition, the data generated glucose gamma model fit is justified by the graphical plots in Figure 1. Figure 1(a) reveals the absolute residuals plot with respect to glucose predicted values, which shows that all absolute residuals are randomly located at a single point except only two residuals. The smooth fitted line is exactly a flat straight line, except the right end which is increasing as a higher residual is located at the right boundary. Figure 1(b) presents the mean normal probability plot of glucose in Table 1. These two plots do not reveal any lack of fit. So, the gamma fitted glucose model (Table 1) is close to its true model. Gamma fitted mean & dispersion models of glucose are as follows.
Gamma fitted glucose mean () model (from Table 1) is
= exp(4.3933 + 0.625 HOMA-IR – 0.128 Insulin + 0.022 Tyes of patients + 0.004 LEPT
+ 0.001 ADIP – 0.002 HOMA-IR*LEPT – 0.001 LEPT*ADIP), and the Gamma fitted glucose variance () model (from Table 1) is
= exp(–8.844 + 2.005 HOMA-IR + 0.053 Age – 0.007 Age*HOMA-IR – 0.162 Insulin + 0.129 REST – 0.039 LEPT – 0.002 Age*REST– 0.001 MCP-1 – 0.011Insulin*HOMA-IR).

From the above glucose fitted mean & dispersion models (i.e., Table 1), the following relationships of glucose with BC markers can be reported.
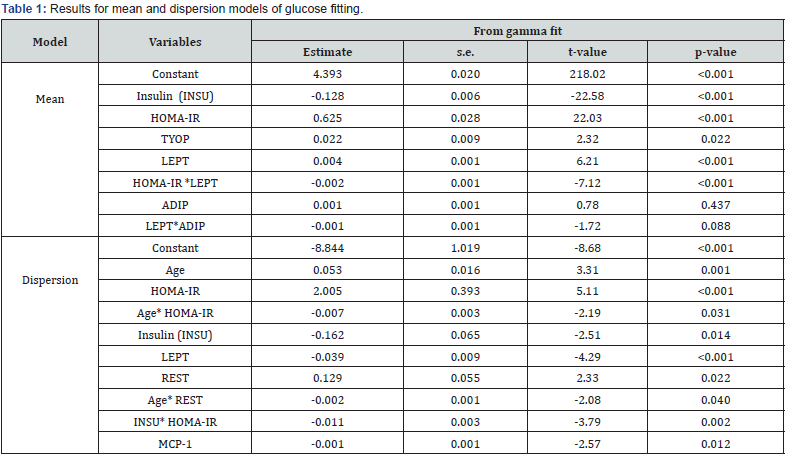
Mean glucose levels are directly related with patient type (P=0.022), interpreting that mean glucose levels are higher for BC women than normal. It concludes that BC women have greater risk of DM. Mean glucose levels are directly related with leptin (P<0.001), implying that mean glucose levels rise as leptin levels increase. Mean glucose levels are directly related with HOMA-IR(P<0.001), concluding that glucose levels rise as HOMAIR levels increase. Mean glucose levels are inversely related to the interaction effect of HOMA-IR and leptin (HOMA-IR*LEPT) (P<0.0001), indicating that mean glucose levels increase as HOMAIR* LEPT decreases. Mean glucose levels are inversely related to the interaction effect of leptin and adiponectin (LEPT*ADIP) (P=0.088), concluding that mean glucose levels rise as the interaction effect LEPT*ADIP decreases. Mean glucose levels are inversely related with Insulin levels (P<0.001), concluding that mean glucose levels rise as insulin levels decrease, which is occurring in practice. Variance of glucose levels is directly related with HOMA-IR (P<0.001), indicating that subjects with higher HOMA-IR levels have highly scattered glucose levels. Variance of glucose levels is directly related with resist in levels (P=0.022), indicating that subjects with higher resistin levels have highly scattered glucose levels.
Variance of glucose levels is inversely related with leptin levels (P<0.001), concluding that subjects with lower leptin levels have highly scattered glucose levels. Variance of glucose levels is inversely related with MCP-1 levels (P=0.012), interpreting that subjects with lower MCP-1 levels have highly scattered glucose levels. Variance of glucose levels is inversely related with Age*HOMA-IR (P=0.031), or INSU* HOMA-IR (P=0.002), or Age*REST (P=0.040), concluding that variance of glucose levels is rises as Age*HOMA-IR, or INSU* HOMA-IR, or Age*REST decreases.
All the above conclusions are drawn from the appropriate glucose levels fitting model (Table 1), where the standard errors of all the estimates (Table 1) are very small, indicating that estimates are stable. From the above it is observed that mean and variance of glucose levels are highly associated with many BC markers and their joint interaction effects. It can be concluded that glucose levels rise for breast cancer women, along with the increase of HOMA-IR, leptin, and decrease of insulin, HOMA-IR*leptin, leptin*adiponectin. From this report medical practitioners and BC women will be benefitted. BC women should always care about their glucose levels regularly.
Conflict of Interest
The authors confirm that this article content has no conflict of interest.
References
- Giovannucci E, Harlan DM, Archer MC, Richard M Bergenstal, Susan M Gapstur, et al. (2010) Diabetes and cancera consensus report. Diabetes Care33(7):1674-1685.
- World Cancer Report (2008) Boyle P, Bernard L, Eds. Cedex, France, World Health Organization, International Agency for Research on Cancer.
- Shao S, Gill AA, Zahm SH, Jatoi I, Shriver CD, et al. (2018) Diabetes and Overall Survival among Breast Cancer Patients in the U.S. Military Health System. Cancer Epidemiol Biomarkers Prev27(1): 50-57.
- Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ (2006) Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet367:1747-1757.
- Kulie T, Slattengren A, Redmer J, Counts H, Eglash A, et al. (2011) Obesity and women's health: an evidence-based review. J Am Board Fam Med24:75-85.
- Azvolinsky A (2014) Cancer prognosis: role of BMI and fat tissue. J Natl Cancer Inst 106:dju177.
- Das RN, Lee Y, Mukherjee S, Oh S (2019) Relationship of body mass index with diabetes & breast cancer biomarkers. J Diabetes and Management9(1):163-168.
- Berrino F, Villarini A, Traina A, Bonanni B, Panico S, et al. (2014) Metabolic syndrome and breast cancer prognosis. Breast Cancer Res Treat147(1):159-165.
- Crisóstomo J, Matafome P, Santos-Silva D, Gomes AL, Gomes M, et al. Hyperresistinemia and metabolic dysregulation: a risky crosstalk in obese breast cancer. Endocrine53(2):433-442.
- Patrício M, Pereira J, Crisóstomo J, Matafome P, Gomes M, et al. (2018) Using Resistin, glucose, age and BMI to predict the presence of breast cancer. BMC Cancer18(1):18-29.
- Lee, Y, Nelder JA, PawitanY (2017) Generalized Linear Models with Random Effects (Unified Analysis via H–likelihood) (second edn). Chapman & Hall, London, UK.
- Das RN, Lee Y (2009) Log-normal versus gamma models for analyzing data from quality-improvement experiments. Quality Engineering 21(1): 79-87.






























