- Case Report
- Abstract
- Diagnosis, pathology and Molecular Biology
- Staging and Risk Assessment
- Treatment
- Postoperative Radiation Therapy is not Recommended in the Postoperative setting
- Follow-up, long-term implications and survival
- MELANOMA. WHAT HAS ASCO 2020 LEFT US?
- Role of Immunotherapy in Neoadjuvant
- References
MELANOMA -Diagnosis, Pathology and Molecular Biology
Dr. Adrian Pablo Hunis*
Recertified Specialist in Oncology, Maimonides University, Argentina
Submission: August 31, 2020; Published:September 28, 2020
*Corresponding Address:Adrian Pablo Hunis, Associate Professor of Internal Medicine (UBA), Director of the Medical Specialist in Oncology (UBA), Professor of Oncology (Maimonides University), Argentina
How to cite this article:Dr. Adrian Pablo Hunis. MELANOMA -Diagnosis, Pathology and Molecular Biology WHAT HAS ASCO 2020 LEFT US?. Canc Therapy & Oncol Int J. 2020; 17(1): 555952.DOI:10.19080/CTOIJ.2020.17.555952
- Case Report
- Abstract
- Diagnosis, pathology and Molecular Biology
- Staging and Risk Assessment
- Treatment
- Postoperative Radiation Therapy is not Recommended in the Postoperative setting
- Follow-up, long-term implications and survival
- MELANOMA. WHAT HAS ASCO 2020 LEFT US?
- Role of Immunotherapy in Neoadjuvant
- References
Abstract
Melanoma is one of the tumors where diagnosis, monitoring and treatment have been most developed with determinations that include the use of molecular biology. New drugs, new combinations of drugs today result in answers that were unthinkable only five years ago. More patients live longer and many of them with advanced disease achieve survival measured in years when years ago we measured it in weeks. In this review we will see the main articles that were presented at ASCO 2020 and what they have left us, to put them into practice for the benefit of our patients.
- Case Report
- Abstract
- Diagnosis, pathology and Molecular Biology
- Staging and Risk Assessment
- Treatment
- Postoperative Radiation Therapy is not Recommended in the Postoperative setting
- Follow-up, long-term implications and survival
- MELANOMA. WHAT HAS ASCO 2020 LEFT US?
- Role of Immunotherapy in Neoadjuvant
- References
Diagnosis, pathology and Molecular Biology
Diagnosis should be based on a full thickness excision biopsy with a small lateral margin.
The histological report must include, as a minimum, information on the following:
• Type of melanoma.
• Actinic damage.
• Maximum vertical thickness in millimeters.
• Information on the rate of mitosis.
• Presentation of ulceration.
• Presence and degree of regression and clear surgical margins.
Testing for treatable mutations is mandatory in patients with resectable or unresectable stage III or stage IV melanoma and is highly recommended for high-risk resected stage IIC melanoma, but not for stage I or stage IIA-IIB melanoma. BRAF testing is mandatory.
- Case Report
- Abstract
- Diagnosis, pathology and Molecular Biology
- Staging and Risk Assessment
- Treatment
- Postoperative Radiation Therapy is not Recommended in the Postoperative setting
- Follow-up, long-term implications and survival
- MELANOMA. WHAT HAS ASCO 2020 LEFT US?
- Role of Immunotherapy in Neoadjuvant
- References
Staging and Risk Assessment
Physical examination is essential with special attention to the following:
• Other suspicious pigmented lesions.
• Tumor satellites.
• Metastasis in transit.
• Metastasis to regional lymph nodes.
• Distant metastasis.
In the more advanced tumor stages, ultrasound, computed tomography or positron emission tomography are recommended for the proper evaluation of the tumor.
- Case Report
- Abstract
- Diagnosis, pathology and Molecular Biology
- Staging and Risk Assessment
- Treatment
- Postoperative Radiation Therapy is not Recommended in the Postoperative setting
- Follow-up, long-term implications and survival
- MELANOMA. WHAT HAS ASCO 2020 LEFT US?
- Role of Immunotherapy in Neoadjuvant
- References
Treatment
For circumscribed disease, local excision of primary tumors is recommended, with the following safety margins:
• Melanomas in situ: 0.5 cm.
• Tumors ≤ 2 mm thick: 1 cm.
• Tumors> 2 mm thick: 2 cm.
For locoregional disease, sentinel lymph node biopsy is recommended in all patients with tumors in stage pT1b or more advanced, according to the staging of the American Joint Committee on Cancer, eighth edition [1].
Complete lymph node dissection is not recommended for patients with positive sentinel node. In the case of isolated metastases to clinically detectable locoregional lymph nodes (macroscopic, no sentinel node), complete lymph node dissection is indicated; it is insufficient to resect only the tumor-bearing lymph node
Patients with resected stage III melanomas should be evaluated for adjuvant (postsurgical) treatment. Postoperative radiation therapy for local tumor control can be considered in cases of inadequate resection margins of lentigo malignant, in R1 resections, or after resection of a bulky tumor.
- Case Report
- Abstract
- Diagnosis, pathology and Molecular Biology
- Staging and Risk Assessment
- Treatment
- Postoperative Radiation Therapy is not Recommended in the Postoperative setting
- Follow-up, long-term implications and survival
- MELANOMA. WHAT HAS ASCO 2020 LEFT US?
- Role of Immunotherapy in Neoadjuvant
- References
Postoperative Radiation Therapy is not Recommended in the Postoperative setting
Preferred treatment options are postsurgical treatment with an anti PD-L1 or dabrafenib / trametinib.
For advanced disease (stages III and IV not resectable), surgical resection or stereotactic radiotherapy of locoregional recurrence or a single distant metastasis should be considered in suitable patients, as a therapeutic option since it offers the possibility of controlling the disease to long term. Patients with metastatic melanoma should be screened for metastasis (preferably) or the primary tumor for detection of the BRAF V600 mutation. The first- and second-line treatment options consist of anti-PD-L1 antibodies (pembrolizumab, nivolumab) with or without ipilimumab for all patients, and the BRAF inhibitor / MEK inhibitor combination for patients presenting with BRAF-mutated melanoma.
Inhibition of antiPD-L1 or an antiPD-L1 plus ipilimumab is now the standard treatment for all patients, regardless of their BRAF expression, in the first-line setting. For NRAS-mutated melanoma, first-line immunotherapy options identical to wildtype melanoma are the first choice, as MEK inhibitors have limited efficacy. If no clinical studies or new approved drugs are available, cytotoxic drugs such as dacarbazine or temozolomide may be administered, with moderate activity expected. For the treatment of brain metastases, the results of studies seem to indicate that the combined treatment with ipilimumab plus nivolumab is the preferred first-line treatment, as well as in asymptomatic patients with BRAF mutation.
For patients with a small number of asymptomatic metastases (<5-10) and non-bulky tumors (<3 cm), stereotactic radiosurgery is initially an option. In other patients, systemic treatment must be evaluated first, reserving stereotactic radiosurgery for the treatment of lesions that do not respond to treatment. In the event that systemic treatment fails, stereotactic radiosurgery could be considered as rescue treatment if the total number of progressive lesions is <5-10, and their maximum size is <3 cm [1].
- Case Report
- Abstract
- Diagnosis, pathology and Molecular Biology
- Staging and Risk Assessment
- Treatment
- Postoperative Radiation Therapy is not Recommended in the Postoperative setting
- Follow-up, long-term implications and survival
- MELANOMA. WHAT HAS ASCO 2020 LEFT US?
- Role of Immunotherapy in Neoadjuvant
- References
Follow-up, long-term implications and survival
To the patients with melanoma, they should be instructed to avoid sunburn and prolonged unprotected exposure to sunlight or artificial ultraviolet light, as well as regular self-examinations of the skin and peripheral lymph nodes for life. Patients should be aware that family members may be at increased risk for melanoma. During melanoma follow-up, patients are monitored clinically for relapses and to recognize additional skin tumors, especially secondary melanomas, as soon as possible. There is no consensus on the optimal monitoring scheme or the usefulness of imaging and blood tests for patients with resected melanoma; Recommendations range from follow-up visits every 3 months in the first 3 years, and every 6 - 12 months thereafter, to no organized follow-up. Patients with sentinel nodes should be followed up with periodic ultrasound examinations. The serum S100 protein test to monitor increases in concentrations is the most accurate blood test for monitoring melanoma patients, if any blood tests are recommended.
- Case Report
- Abstract
- Diagnosis, pathology and Molecular Biology
- Staging and Risk Assessment
- Treatment
- Postoperative Radiation Therapy is not Recommended in the Postoperative setting
- Follow-up, long-term implications and survival
- MELANOMA. WHAT HAS ASCO 2020 LEFT US?
- Role of Immunotherapy in Neoadjuvant
- References
MELANOMA. WHAT HAS ASCO 2020 LEFT US?
EORTC 1325-MG / Keynote-054 is a phase 3 study that randomized fully resected stage III melanoma patients to receive 200 mg fixed-dose pembrolizumab every 3 weeks for one year or placebo. In this update at 3 years of follow-up, pembrolizumab confirmed its benefit in progression-free survival with 63.7% (95% CI: 59.2 - 67.7) of living patients and without recurrence compared to 44.1% ( 95% CI: 39.6 - 48.4) of those treated with placebo (HR: 0.56; 95% CI: 0.47 - 0.68; p <0.001) [2].
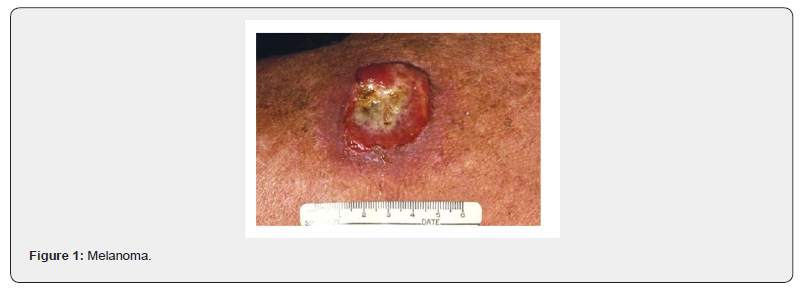
The benefit was independent of PD-L1 status (ligand 1 of programmed cell death), clinical stage (IIIA vs. IIIB vs. IIIC), and BRAF status. The most frequent site of recurrence was distant metastases and the incidence of distant metastases was higher in the placebo group compared to that of pembrolizumab (HR: 0.55, 95% CI 0.44 - 0.69, p < 0.001). Distant metastasis-free survival analysis is expected in late 2020 (Figure 1).
The authors of the COMBI-AD study also presented an update of their study with a 5-year follow-up [2]. This phase 3 clinical study randomized patients with clinical stage III melanoma and BRAF V600E / K mutations to receive dabrafenib (150 mg every 12 hours) and trametinib (2 mg every day) or placebo for one year. At 5 years of follow-up, the median relapse-free survival with dabrafenib plus trametinib has not been reached (95% CI: 47.9- not reached), and that of placebo is 16.6 (95% CI: 12, 7 - 22.1) months (52% of patients alive and without recurrence [95% CI: 48% - 58%] for dabrafenib plus trametinib; 36% [95% CI: 32% - 41%] for placebo: HR: 0.51 [95% CI: 0.42-0.61]). The results were independent of the type of BRAF mutation and the clinical stage. The 5-year distant metastasis-free survival is 65% (95% CI: 61% - 71%) for the dabrafenib plus trametinib group vs. 54% (95% CI: 49% - 60%) in the group. placebo (HR: 0.55, 95% CI 0.44 - 0.70). The median distance metastasis-free survival has not been reached for either group.
These studies confirm the utility of both immunotherapy and target therapies for the adjuvant treatment of patients with clinical stage III melanoma. Some important questions remain open, such as the benefit of these treatments in clinical stage IIIA, which seems to be bordering on both studies. On the other hand, the question arises as to which is the best therapy for patients with BRAF mutations; This question remains unanswered, and for the time being the decision will have to be based on the toxicity profile and the preference of both the physician and the patient.
- Case Report
- Abstract
- Diagnosis, pathology and Molecular Biology
- Staging and Risk Assessment
- Treatment
- Postoperative Radiation Therapy is not Recommended in the Postoperative setting
- Follow-up, long-term implications and survival
- MELANOMA. WHAT HAS ASCO 2020 LEFT US?
- Role of Immunotherapy in Neoadjuvant
- References
Role of Immunotherapy in Neoadjuvant
Although the results of the PRADO study do not change our clinical practice at this time, they suggest that there will be a benefit of immunotherapy in the neoadjuvant field. This phase 2 study analyzes personalized treatment for patients with clinical stage IIIB and IIIC melanoma, without transit metastases. Previously, the results of several studies in this treatment context had been reported; In particular, the results of the Neo-OPACIN study demonstrated adequate tolerance and safety for a combination of ipilimumab at a dose of 1 mg / kg with nivolumab at a dose of 3 mg / kg (ipilimumab plus nivolumab), with high response rates.
In the PRADO study, patients received ipilimumab plus nivolumab for 2 cycles, and resection of the index adenopathy, arguing that the pathological response in the largest infiltrated lymph node represents the state of the entire lymph bed in question. If after treatment with ipilimumab plus nivolumab the index adenopathy presented a complete pathological response or less than or equal to 10% of viable tumor cells, only surveillance was given. If there was a partial response, between 10% and 50% of viable tumor cells, it was carried out to lymph node dissection and then to surveillance. If there was no adequate pathological response, adjuvant lymph node dissection and nivolumab, or dabrafenib plus trametinib (in the case of a BRAF mutation) were carried out.
The study reported 61% complete pathologic response or viable tumor cells after 2 cycles of ipilimumab plus nivolumab, and 21% adequate pathologic response. The radiological evaluation, on the other hand, reported an objective response rate (ORR) of only 45%, therefore it was concluded that the radiological response underestimated the pathological evaluation. Grade 3-4 adverse events secondary to ipilimumab plus nivolumab were 22%. There were greater postoperative complications in patients who underwent lymph node dissection, compared to those who were led only to index lymph node dissection (81% vs. 41%, respectively; p <0.001). The patients who only had resection of index adenopathy also had a better quality of life than those who had complete resection. The results of recurrence-free survival and distant metastasis-free survival will be presented at the 2020 European Society for Medical Oncology (ESMO) Congress.
Despite being a small phase 2 study, it brings up various concepts that we will have to take into account for neoadjuvant treatment: 1) 2 cycles of ipilimumab plus nivolumab are probably sufficient to achieve high pathological response rates; 2) Imaging-assessed responses are likely to underestimate pathologic response rates, and 3) bring to the table the concept of the index lymph node, which could prevent wide lymph node dissections that have a detrimental effect on the quality of life of patients. We will wait impatiently for the results of the outcomes over time, and of course, phase 3 studies that confirm the value of immunotherapy in this context of the disease. Palliative land, one of the greatest needs for the progression of treatment with immunotherapy.
A phase 2 study evaluated the efficacy of the combination of pembrolizmab with ipilimumab at a dose of 1 mg / kg body weight (ipilimumab plus pembrolizmab) for 4 doses, followed by maintenance with pembrolizumab in patients with metastatic melanoma with progression to treatment with an anti- PD1 or a combination of anti-PD1 with another non-CTLA4 immunotherapy agent.
The RECIST response rates were 27%, with a response duration of 18.5 months (95% CI: 10.6-undetermined). The median progression-free survival was 5 months, and the overall survival 24.7 months. Grade 3-4 adverse effects occurred in 27% of patients. The response was variable between patients treated with the combo and those with higher response rates were those with liver or central nervous system disease, elevated lactic dehydrogenase, and negative PD-L1. In a gene expression profile analysis, the highest efficacy was observed in non-T-cell inflamed tumors. This benefit was confirmed in a retrospective analysis of patients with progression to adjuvant or palliative immunotherapy, looking at the benefit of using a combination of ipilimumab monotherapy versus ipilimimumab plus anti-PD1 in patients previously treated with an anti-PD1.
In this cohort, 88% of the patients had been treated in the metastatic context; 74% of them were classified as primary resistances (median progression: 2.7 months), and 26% as acquired resistances (median progression: 9.5 months). There were significant differences in the general characteristics of the patients who were treated with ipilimimumab compared to those treated with ipilimimumab plus anti-PD1. In general, the patients who received the combo were younger, had more frequent BRAF mutations, better ECOG (<1), and more frequent presence of brain metastases than patients treated with ipilimimumab.
Objective RECIST response rates for patients treated with ipilimimumab plus anti-PD1 were 27% versus 13% for those treated with ipilimimumab (p = 0.0021), with a median duration of response of 11.6 months versus 9 months, respectively (p = 0.04). The progression-free survival at 18 months was 22% for the patients treated with the combo compared to 18% of those treated with ipilimimumab (HR: 0.67, 95% CI: 0.53 - 0.85; p = 0 , 0005), and the overall survival of 53% versus 25%, respectively (HR: 0.51; 95% CI: 0.36 - 0.67; p <= 0.0001). In particular, BRAF WT patients had better response rates with ipilimimumab plus anti-PD1, than with ipilimimumab.
Predictive factors associated with longer survival in the combo-treated cohort were male gender, time to progression with previous anti-PD1 greater than 3 months, and treatment with ipilimimumab plus anti-PD1. Negative predictive factors for overall survival were ECOG less than or equal to 1, presence of bone metastases and elevated lactic dehydrogenase. Grade 3-4 adverse events occurred in 31% of patients treated with the combo, and in 33% of those treated with monotherapy. These data are the first prospective and randomized results that we have in patients with metastatic melanoma with progression to a previous anti-PD1. These, plus the results of several retrospective cohorts, confirm the potential benefit of this strategy in an area of therapeutic need. Furthermore, a more specific clinical profile seems to be being defined on the patients who will obtain the greatest benefit from this combination.
- Case Report
- Abstract
- Diagnosis, pathology and Molecular Biology
- Staging and Risk Assessment
- Treatment
- Postoperative Radiation Therapy is not Recommended in the Postoperative setting
- Follow-up, long-term implications and survival
- MELANOMA. WHAT HAS ASCO 2020 LEFT US?
- Role of Immunotherapy in Neoadjuvant
- References
References
- Huñis Adrian P (2010) Introduction to Clinical Oncology. Editorial of the National University of Quilmes. Vol II.
- Virtual Congress of the American Society of Clinical Oncology (ASCO) 2020.






























