Target Volume Definition for Stereotactic Radiosurgery (SRS) Of Cerebral Cavernous Malformations (CCMS)
Omer Sager*, Selcuk Demiral, Ferrat Dincoglan and Murat Beyzadeoglu
Department of Radiation Oncology; University of Health Sciences, Gulhane Medical Faculty, Turkey
Submission: January 09, 2020; Published: January 31, 2020
*Corresponding Address: Omer Sager, University of Health Sciences, Gulhane Medical Faculty, Department of Radiation Oncology, Gn.Tevfik Saglam Cad. 06018, Etlik, Kecioren, Ankara, Turkey
How to cite this article: Omer Sager, Selcuk Demiral, Ferrat Dincoglan, Murat Beyzadeoglu. Target Volume Definition for Stereotactic Radiosurgery (SRS) Of Cerebral Cavernous Malformations (CCMS). Canc Therapy & Oncol Int J. 2020; 15(4): 555917. DOI:10.19080/CTOIJ.2020.15.555917
Abstract
Objective: Cerebral cavernous malformations (CCMs) are composed of abnormal hyalinized capillary clusters typically surrounded by deposits of hemosiderin. These vascular abnormalities of the brain may be asymptomatic, however, a plethora of symptoms may occur in some of the affected patients including seizures, hemorrhages, and neurological deficits. Stereotactic radiosurgery (SRS) has been utilized as a noninvasive modality of management for selected patients with high risk CCMs located at eloquent brain regions typically not amenable to surgical removal. In this study, we assessed incorporation of multimodality imaging into target volume definition of CCM radiosurgery
Materials and methods: Twenty-three patients treated with SRS for CCM at our institution were included. Target definition with CT only and by incorporation of CT-MR fusion was comparatively evaluated.
Results: Twenty-three patients receiving SRS for CCMs at our institution were evaluated for target volume determination using CT-only imaging and CT-MR fusion based imaging. Ground truth target volume defined by treating physicians after comprehensive assessment and consensus was identical to target definition using CT-MR fusion based imaging in the majority of patients.
Conclusion: MRI may be utilized for improving the definition of SRS target for CCM management.
Keywords: Cerebral Cavernous Malformation (CCM); Stereotactic radiosurgery (SRS); Magnetic Resonance Imaging (MRI); Target volume definition
Introduction
Cerebral cavernous malformations (CCMs), also referred to as cavernous angioma, hemangioma, or cavernoma, are composed of abnormal hyalinized capillary clusters typically surrounded by deposits of hemosiderin [1-3]. These vascular abnormalities of the brain may be asymptomatic, however, a plethora of symptoms may occur in some of the affected patients including seizures, hemorrhages, and neurological deficits with the potential of a substantial decline in the general health status and quality of life [4,5]. Primary objective of CCM treatment is reduction of the hemorrhage risk. Surgery is a viable therapeutic option for elimination of future risk of bleeding from CCMs [6,7]. From a radiation oncology standpoint, radiosurgery has emerged as a viable treatment modality for management of several intracranial and extracranial benign and malign conditions [8-30]. In the context of CCMs, radiosurgery has been utilized as a noninvasive modality of management for selected patients with high risk CCMs located at eloquent brain regions typically not amenable to surgical removal. While reduction in hemorrhage risk with radiosurgery is a pertinent goal of treatment, precise target definition is an indespensable part of radiosurgical management to achieve a favorable toxicity profile and treatment outcome. Multimodality imaging has been integrated into target definition for CCMs to achieve precise radiosurgical treatment under robust immobilization and image guidance. In this study, we assessed incorporation of multimodality imaging into target volume definition of CCM radiosurgery.
Materials and Methods
A total of 23 patients treated with Stereotactic Radiosurgery (SRS) for CCM at our institution were identified and included in this study. Informed consents of all patients were obtained before SRS, and management of patients with radiosurgery was decided by multidisciplinary collaboration of experts from neurosurgery, neuroradiology, and radiation oncology after evaluation of lesion size, location, symptomatology, presenting symptom and performance status, patient age and preferences. On the day of SRS, a stereotactic head frame was affixed to the patients’ skull under local anesthesia by use of 4 pins. The patients were then simulated at Computed Tomography (CT) simulator (GE Lightspeed RT, GE Healthcare, Chalfont St. Giles, UK) available at our institution using a slice thickness of 1.25 mm. Acquired planning CT images were transferred to the delineation workstation (SimMD, GE, UK) for contouring of the target volumes and critical structures in close vicinity. Target volume definition for SRS was based on CT simulation images only or fused CT and T1 gadoliniumenhanced MR images typically acquired the day before SRS. Target definition with CT only and by incorporation of CT-MR fusion was comparatively evaluated. Definition of ground truth target volume for actual treatment and comparison purposes was performed by consensus of treating physicians after comprehensive assessment and colleague peer review. ERGO ++ (CMS, Elekta, UK) radiosurgery planning system and Synergy (Elekta, UK) Linear Accelerator (LINAC) was used for SRS planning and delivery, respectively. Median prescribed dose for radiosurgery was 15 Gy (range: 10-20 Gy) to the 85%-95% isodose line encompassing the target volume. Image Guided Radiation Therapy (IGRT) techniques such as kV-CBCT (kilovoltage Cone Beam CT) and XVI (X-ray Volumetric Imaging, Elekta, UK) were used for treatment verification. All patients received dexamethasone with H2- antihistamines routinely after SRS.
Results
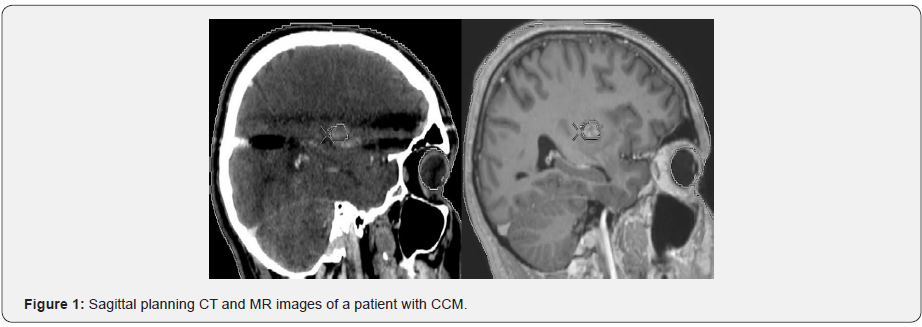
Twenty-three patients receiving SRS for CCMs at our institution were evaluated for target volume determination using CT-only imaging and CT-MR fusion based imaging. Ground truth target volume defined by treating physicians after comprehensive assessment and consensus was identical to target definition using CT-MR fusion based imaging in the majority of patients. Contouring of target volume on the planning CT and MR images was optimized through selecting appropriate windows and levels in SRS treatment planning. Delineation accuracy was improved by using the coronal and sagittal images along with the axial planning CT images. Optimization of target volume coverage and normal tissue sparing was achieved by use of the Arc Modulation Optimization Algorithm (AMOA). Single session SRS was performed using the Elekta Synergy LINAC with 6-MV photons available at our institution. Sagittal planning CT and MR images of a patient with CCM are shown in Figure 1.
Discussion
The role of SRS in management of CCMs is still being refined. Surgery remains to be the primary mode of treatment with complete elimination of future bleeding risk. However, surgical resection may not be preferred in selected patients due to critical location of some lesions at eloquent brain regions in intimate association with vital neurovascular structures. Alternative therapeutic strategies are considered when there is excessive risk of surgical complications. In this context, SRS has emerged as a viable treatment alternative for selected patients with CCMs [17,31-34]. Given the hazards of rebleeding from a CCM with previous hemorrhage history, a more active approach with radiosurgical management of surgically inaccessible deep-seated CCMs has been suggested in the literature [35-37]. Nevertheless, treatment with radiosurgery may also cause untoward toxicity leading to deterioration in the patients’ quality of life. From this aspect, target volume determination for SRS of CCMs becomes more critical. MRI may add to the accuracy of target definition in SRS of CCM by providing valuable information. CCMs are typically surrounded by a hypointense ring resulting from hemosiderin deposits of microhemorrhages [38-40]. MRI substantially facilitates detection of incidental CCMs, by demonstrating a reticulated pattern including mixed hyperintensity and hypointensity on T1 and T2 weighted sequences along with a typical hypointense rim visualized on gradient-echo or T2 weighted imaging.
Developmental vascular anomalies may be detected by use of contrast enhanced MRI. Screening of familial CCMs may be performed by using susceptibility weighted MRI. Utility of neuroimaging with MRI for determination of central nervous system radiotherapy and radiosurgery target volumes has been addressed in the literature [41-49]. In the context of CCM radiosurgery, MRI improves precision in target definition by producing additional imaging data for accurate target localization. The ground truth target volumes defined after comprehensive assessment, collaboration and consensus of treating physicians were found to be identical to target volumes defined based on CT-MR fusion based imaging in majority of patients in our study, supporting the incorporation of MRI in radiosurgery treatment planning.
In conclusion, MRI may be used to improve target definition for SRS of CCMs. Clearly, future studies are required for assessing the utility of multimodality imaging for radiosurgery target volume definition for radiosurgical management of CCMs.
References
- Raychaudhuri R, Batjer HH, Awad IA (2005) Intracranial cavernous angioma: a practical review of clinical and biological aspects. Surg Neurol 63(4): 319-328.
- Akers A, Al-Shahi Salman R, A Awad I, Dahlem K, Flemming K, et al. (2017) Synopsis of Guidelines for the Clinical Management of Cerebral Cavernous Malformations: Consensus Recommendations Based on Systematic Literature Review by the Angioma Alliance Scientific Advisory Board Clinical Experts Panel. Neurosurgery 80(5): 665-680.
- Yadla S, Jabbour PM, Shenkar R, Shi C, Campbell PG, et al. (2010) Cerebral cavernous malformations as a disease of vascular permeability: from bench to bedside with caution. Neurosurg Focus 29(3): E4.
- Mouchtouris N, Chalouhi N, Chitale A, Starke RM, Tjoumakaris SI, et al. (2015) Management of cerebral cavernous malformations: from diagnosis to treatment. Scientific World Journal 2015: 808314.
- Al-Shahi Salman R, Hall JM, Horne MA, Moultrie F, Josephson CB, et al. (2012) Untreated clinical course of cerebral cavernous malformations: a prospective, population-based cohort study. Lancet Neurol 11(3): 217-224.
- Flemming KD (2017) Clinical Management of Cavernous Malformations. Curr Cardiol Rep 19(12): 122.
- Gross BA, Du R (2015) Cerebral cavernous malformations: natural history and clinical management. Expert Rev Neurother 15(7): 771-777.
- Dincoglan F, Sager O, Demiral S, Gamsiz H, Uysal B, et al. (2019) Fractionated stereotactic radiosurgery for locally recurrent brain metastases after failed stereotactic radiosurgery. Indian J Cancer 56(2): 151-156.
- Sirin S, Oysul K, Surenkok S, Sager O, Dincoglan F, et al. (2011) Linear accelerator-based stereotactic radiosurgery in recurrent glioblastoma: A single center experience. Vojnosanit Pregl 68(11): 961-966.
- Dincoglan F, Beyzadeoglu M, Sager O, Oysul K, Sirin S (2012) Image-guided positioning in intracranial non-invasive stereotactic radiosurgery for the treatment of brain metastasis. Tumori 98(5): 630-635.
- Dincoglan F, Sager O, Gamsiz H, Demiral S, Uysal B (2012) Management of arteriovenous malformations by stereotactic radiosurgery: A single center experience. UHOD-Uluslararasi Hematoloji-Onkoloji Dergisi 22(4): 107-112.
- Surenkok S, Sager O, Dincoglan F, Gamsiz H, Demiral S (2012) Stereotactic radiosurgery in pituitary adenomas: A single center experience. UHOD-Uluslararasi Hematoloji-Onkoloji Dergisi 22: 255-260.
- Dincoglan F, Sager O, Gamsiz H, Uysal B, Demiral S, et al. (2012) Stereotactic radiosurgery for intracranial tumors: A single center experience. Gulhane Med J 54(3): 190-198.
- Sager O, Beyzadeoglu M, Dincoglan F, Demiral S, Uysal B, et al. (2013) Management of vestibular schwannomas with linear accelerator-based stereotactic radiosurgery: A single center experience. Tumori 99(5): 617-622.
- Dincoglan F, Beyzadeoglu M, Sager O, Uysal B, Demiral S, et al. (2013) Evaluation of linear accelerator-based stereotactic radiosurgery in the management of meningiomas: A single center experience. J BUON 18(3): 717-722.
- Demiral S, Beyzadeoglu M, Uysal B, Oysul K, Kahya YE, et al. (2013) Evaluation of stereotactic body radiotherapy (SBRT) boost in the management of endometrial cancer. Neoplasma 60(3): 322-327.
- Sager O, Beyzadeoglu M, Dincoglan F, Uysal B, Gamsiz H, et al. (2014) Evaluation of linear accelerator (LINAC)-based stereotactic radiosurgery (SRS) for cerebral cavernous malformations: A 15-year single-center experience. Ann Saudi Med 34(1): 54-58.
- Sager O, Beyzadeoglu M, Dincoglan F, Gamsiz H, Demiral S, et al. (2014) Evaluation of linear accelerator-based stereotactic radiosurgery in the management of glomus jugulare tumors. Tumori 100(2): 184-188.
- Sager O, Dincoglan F, Beyzadeoglu M (2015) Stereotactic radiosurgery of glomus jugulare tumors: Current concepts, recent advances and future perspectives. CNS Oncol 4(2): 105-114.
- Gamsiz H, Beyzadeoglu M, Sager O, Dincoglan F, Demiral S, et al. (2014) Management of pulmonary oligometastases by stereotactic body radiotherapy. Tumori 100(2): 179-183.
- Dincoglan F, Sager O, Gamsiz H, Uysal B, Demiral S, et al. (2014) Management of patients with ≥ 4 brain metastases using stereotactic radiosurgery boost after whole brain irradiation. Tumori 100(3): 302-306.
- Demiral S, Beyzadeoglu M, Sager O, Dincoglan F, Gamsiz H, et al. (2014) Evaluation of linear accelerator (linac)-based stereotactic radiosurgery (srs) for the treatment of craniopharyngiomas. UHOD - Uluslararasi Hematoloji-Onkoloji Dergisi 24: 123-129.
- Gamsiz H, Beyzadeoglu M, Sager O, Demiral S, Dincoglan F, et al. (2015) Evaluation of stereotactic body radiation therapy in the management of adrenal metastases from non-small cell lung cancer. Tumori 101(1): 98-103.
- Dincoglan F, Beyzadeoglu M, Sager O, Demiral S, Gamsiz H, et al. (2015) Management of patients with recurrent glioblastoma using hypofractionated stereotactic radiotherapy. Tumori 101(2): 179-184.
- Demiral S, Dincoglan F, Sager O, Gamsiz H, Uysal B, et al. (2016) Hypofractionated stereotactic radiotherapy (HFSRT) for who grade I anterior clinoid meningiomas (ACM). Jpn J Radiol 34(11): 730-737.
- Dincoglan F, Sager O, Demiral S, Uysal B, Gamsiz H, et al. (2017) Radiosurgery for recurrent glioblastoma: A review article. Neurol Disord Therap 1: 1-5.
- Demiral S, Dincoglan F, Sager O, Uysal B, Gamsiz H, et al. (2018) Contemporary Management of Meningiomas with Radiosurgery. Int J Radiol Imaging Technol 80(2): 187-190.
- Dincoglan F, Sager O, Uysal B, Demiral S, Gamsiz H, et al. (2019) Evaluation of hypofractionated stereotactic radiotherapy (HFSRT) to the resection cavity after surgical resection of brain metastases: A single center experience. Indian J Cancer 56(3): 202-206.
- Sager O, Dincoglan F, Demiral S, Uysal B, Gamsiz H (2018) A concise review of immunotherapy for glioblastoma. Neuroimmunol Neuroinflammation 5: 25.
- Sager O, Dincoglan F, Demiral S, Uysal B, Gamsiz H, et al. (2018) Radiation Therapy (RT) for Diffuse Intrinsic Pontine Glioma (DIPG) in Children. Arch Can Res 6(3): 14.
- Liu HB, Wang Y, Yang S, Gong FL, Xu YY, et al. (2016) Gamma knife radiosurgery for brainstem cavernous malformations. Clin Neurol Neurosurg 151: 55-60.
- Chalouhi N, Jabbour P, Andrews DW (2013) Stereotactic radiosurgery for cavernous malformations: is it effective? World Neurosurg 80(6): e185-186.
- López-Serrano R, Martínez NE, Kusak ME, Quirós A, Martínez R (2017) Significant Hemorrhage Rate Reduction after Gamma Knife Radiosurgery in Symptomatic Cavernous Malformations: Long-Term Outcome in 95 Case Series and Literature Review. Stereotact Funct Neurosurg 95(6): 369-378.
- Lunsford LD, Khan AA, Niranjan A, Kano H, Flickinger JC, et al. (2010) Stereotactic radiosurgery for symptomatic solitary cerebral cavernous malformations considered high risk for resection. J Neurosurg 113(1): 23-29.
- Nagy G, Kemeny AA (2015) Radiosurgery for cerebral cavernomas. J Neurosurg Sci 59(3): 295-306.
- Lee SH, Choi HJ, Shin HS, Choi SK, Oh IH, et al. (2014) Gamma Knife radiosurgery for brainstem cavernous malformations: should a patient wait for the rebleed? Acta Neurochir (Wien) 156(10): 1937-1946.
- Nagy G, Razak A, Rowe JG, Hodgson TJ, Coley SC, et al. (2010) Stereotactic radiosurgery for deep-seated cavernous malformations: a move toward more active, early intervention. Clinical article. J Neurosurg 113(4): 691-699.
- de Champfleur NM, Langlois C, Ankenbrandt WJ, Le Bars E, Leroy MA, et al. (2011) Magnetic resonance imaging evaluation of cerebral cavernous malformations with susceptibility-weighted imaging. Neurosurgery 68(3): 641-647.
- Wang KY, Idowu OR, Lin DDM (2017) Radiology and imaging for cavernous malformations. Handb Clin Neurol 143: 249-266.
- Campbell PG, Jabbour P, Yadla S, Awad IA (2010) Emerging clinical imaging techniques for cerebral cavernous malformations: a systematic review. Neurosurg Focus 29(3): E6.
- Demiral S, Sager O, Dincoglan F, Uysal B, Gamsiz H, et al. (2018) Evaluation of Target Volume Determination for Single Session Stereotactic Radiosurgery (SRS) of Brain Metastases. Canc Therapy & Oncol Int J 12(5): 555848.
- Sager O, Dincoglan F, Demiral S, Gamsiz H, Uysal B, et al. (2019) Evaluation of the Impact of Magnetic Resonance Imaging (MRI) on Gross Tumor Volume (GTV) Definition for Radiation Treatment Planning (RTP) of Inoperable High Grade Gliomas (HGGs). Concepts in Magnetic Resonance Part A 2019: 4282754.
- Sager O, Dincoglan F, Demiral S, Gamsiz H, Uysal B, et al. (2019) Utility of Magnetic Resonance Imaging (Imaging) in Target Volume Definition for Radiosurgery of Acoustic Neuromas. Int J Cancer Clin Res 6: 119.
- Demiral S, Sager O, Dincoglan F, Beyzadeoglu M (2019) Assessment of target definition based on Multimodality imaging for radiosurgical Management of glomus jugulare tumors (GJTs). Cancer Ther Oncol Int J 15(2): CTOIJ.MS.ID.555909.
- Dincoglan F, Sager O, Demiral S, Beyzadeoglu M (2019) Multimodality Imaging for Radiosurgical Management of Arteriovenous Malformations. Asian Journal of Pharmacy, Nursing and Medical Sciences 7(1): 7-12.
- Sager O, Dincoglan F, Demiral S, Beyzadeoglu M (2019) Evaluation of Radiosurgery Target Volume Determination for Meningiomas Based on Computed Tomography (CT) And Magnetic Resonance Imaging (MRI). Cancer Sci Res Open Access 5(2): 1-4.
- Beyzadeoglu M, Sager O, Dincoglan F, Demiral S. (2019) Evaluation of Target Definition for Stereotactic Reirradiation of Recurrent Glioblastoma. Arch Can Res 7(1): 3.
- Demiral S, Sager O, Dincoglan F, Beyzadeoglu M (2019) Assessment of Computed Tomography (CT) And Magnetic Resonance Imaging (MRI) Based Radiosurgery Treatment Planning for Pituitary Adenomas. Canc Therapy & Oncol Int J 13(2): CTOIJ.MS.ID.555857.
- Dincoglan F, Sager O, Demiral S, Beyzadeoglu M (2019) Incorporation of Multimodality Imaging in Radiosurgery Planning for Craniopharyngiomas: An Original Article. SAJ Cancer Sci 6: 103.






























