Antidepressant Drug Sertraline up-regulates Death Receptor 5 to attenuate TRAIL Resistance in Lung Cancer via Inhibition of Autophagy Flux
K M A Zinnah1,2K M A Zinnah1*
1Biosafety Research Institute, College of Veterinary Medicine, Chonbuk National University, South Korea
2Faculty of Biotechnology and Genetic Engineering, Department of Animal and Fish Biotechnology, Bangladesh
Submission: September 23, 2019; Published: October 18, 2019
*Corresponding Address: Sang-Youel Park, College of Veterinary Medicine, Chonbuk National University, Gobong ro, Iksan, Jeonbuk 54596, South Korea
How to cite this article:Lung cancer is one of the most common cancers reported. It is the leading cause of death worldwide. Cancer research and clinical trials are ongoing to develop cancer treatment methodology [1-3]. Canc Therapy & Oncol Int J. 2019; 15(1): 555905. DOI:10.19080/CTOIJ.2019.15.555905
Abstract
Depression is very common in cancer patients. A life enormously fluctuates after cancer diagnosis, and considerably placed psychological and emotional stress. Sertraline is an antidepressant drug that also possesses anticancer properties. It is widely used for major depressive disorder, obsessive–compulsive disorder, panic disorder, and social anxiety disorder.
Keywords: Sertraline, TRAIL, Death receptor-5, Apoptosis, Autophagy
Introduction
Lung cancer is one of the most common cancers reported. It is the leading cause of death worldwide. Cancer research and clinical trials are ongoing to develop cancer treatment methodology [1-3]. There are several anticancer treatments available for cancer patients, including surgery, radiotherapy, chemotherapeutic drugs, or their combinations. For advanced progressive tumors that are resistant to monotherapy, combination therapy may augment the regression of specific types of tumors even at progressive stages. Combination strategy has played an important role in cancer treatment for several years. In addition, combination strategies with potent chemotherapeutic drugs can exert prospective advantages against cancers such as non-small cell lung adenocarcinoma (NSCLC) [4-7]. Tumor necrosis factor related apoptosis inducing ligand (TRAIL) is a well known transmembrane cytokine that can selectively induce cancer cell death by binding to death receptors on cell membrane with negligible toxicity to normal cells [8,9].
TRAIL can selectively kill cells that are proliferating uncontrollably without harming normal cells. Furthermore, many cancer cells are resistant to TRAIL via different mechanisms, including genetic or epigenetic modification of TRAIL receptors, increased expression of decoy receptors, and decreased expression of DR4/DR5 [10-13]. Binding of TRAIL to death receptors may also involve the extrinsic apoptotic pathway and activate apoptotic signaling [14]. TRAIL can bind to its receptors DR4 and DR5 to form death-inducing signaling complexes (DISCs). DISCs are associated with adaptor molecules FADD and caspase- 8, causing activation of caspase-9 and consequently activating caspase-3 to cause apoptotic cell death [15-18]. It has been reported that a number of cancer cells, including lung A549 cells, are resistant to apoptotic effects of TRAIL [19]. Interestingly, TRAIL resistance can be overcome by using efficient TRAIL-sensitizing pharmacological agents [20,21].
Selective serotonin reuptake inhibitors (SSRIs) are widely used to treat depression, anxiety, and certain behavioral disorders. They are also prescribed frequently for treating depression of cancer patients [22,23]. Anti-tumor effects of several SSRIs against many types of cancer cells have been described. Paroxetine, an SSRI, can induce death of human osteosarcoma cells through apoptosis by activating p38 mitogenactivated protein kinase (MAPK) and caspase-3 pathways [24]. Fluoxetine, another SSRI, can inhibit the proliferation of prostate cancer cells both in vitro and in vivo and lead to apoptosis of glioma cells [25,26]. In addition, SSRIs sertraline and paroxetine can increase the activity of caspase-3, decrease the expression of Bcl-2, and significantly decrease the viability of malignant T cells [27]. Sertraline is a broadly used antidepressant drug. It possesses antitumor activity in a variety of cancers, including liver cancer, colorectal cancer, and lymphoma [28].
Autophagy flux inhibition may be an applicable and effective technique for cancer treatment. Chloroquine (CQ) and 3-methyladenine (3-MA) are commonly used as autophagy inhibitors in autophagy studies. Chloroquine (CQ) inhibits lysosome acidification and lysosomal fusion with autophagosomes. It also hinders the degradation of metabolic stress, thereby inducing apoptosis [40,41]. 3-methyladenine (3-MA) is a specific inhibitor of PI3K and autophagy [42]. AMPactivated protein kinase (AMPK), a key energy-preserving intracellular enzyme, regulates energy balance to maintain cellular energy homeostasis. Activation of AMPK induces autophagy through consequent inhibition of mTOR under several stress conditions in cellular environment. Many studies have revealed that downstream of AMPK phosphorylation provides anticancer effect while autophagy plays a cell survival role in anticancer mechanisms [43-45].
The present study intended to explore the use of sertraline as a sensitizing agent to TRAIL‑initiated apoptosis in A549 cells. Thus, we investigated molecular mechanisms underlying the anticancer effect of sertraline combined with TRAIL treatment that could inhibit autophagy via blocking of AMPK phosphorylation, prompting DR5 activation, providing effective chemotherapeutic properties, and attenuating TRAIL resistance in lung cancer.
Materials and Methods
Cell culture
Cancer cells A549 and HCC-15originating from lung tumors were acquired from the American Type Culture Collection (Global Bioresource Center, Manassas, VA, USA). Calu-3 cancer cells were acquired from Korean Cancer Cell Bank (KCBL), Republic of Korea. Cells were cultured in RPMI- 1640 medium (Gibco BRL, Grand Island, NY, USA) supplemented with 10% (v/v) fetal bovine serum and antibiotics (100 μg/ml penicillinstreptomycin) at 37 °C in a 5% CO2 incubator
Reagents
Sertraline, a selective serotonin reuptake inhibitor, was purchased from Cayman chemical (Ann Arbor, MI, USA). Chloroquine (10 μM), 3-MA (5 mM) was obtained from Sigma- Aldrich (St. Louis, MO, USA). TRAIL (100 ng/ml) was purchased from Abfrontier (Geumcheon-gu, Seoul, South Korea).
Cell Viability Test
A549, HCC-15, and Calu-3 cells were plated into 12-well plates at a density of 1.0×104 cells and incubated at 37 °C for 24 h. After A549 cells were pretreated with sertraline at different concentrations (0, 2.5, 5, and 10 μM) for 18 h, recombinant TRAIL protein (100 ng/ml) was added and co-incubated for 2.3 h. Additionally, cells were pretreated with chloroquine (10 μM) for 1 h before sertraline treatment. Cell morphology was observed under an inverted microscope (Nikon, Japan). Cell viability was assessed by using the crystal violet staining method. In this method, cells were stained with crystal violet staining solution (0.5% crystal violet in 30% ethanol and 3% formaldehyde) for 10-15 min at room temperature, washed 3-4 times with phosphate buffered saline (PBS), and dried. Cell viability was also measured by adding 50 μl of 5 mg/ml methylthiazolyl- tetrazolium (MTT) to each well of the plate followed by incubation at 37 °C for 2 h. After removing the old media, 500 μl of dimethyl sulfoxide (DMSO) was added to each well and absorbance was measured at 570 nm with a spectrophotometer (Bio-Rad, Hercules, CA, USA).
Lactate Dehydrogenase Assay
Cytotoxicity from cell supernatants was determined using a lactate dehydrogenase (LDH) cytotoxicity detection kit (Takara Bio, Inc., Tokyo, Japan) according to the manufacturer’s protocol. LDH activity was assessed by measuring absorbance at 490 nm using a microplate reader (Spectra Max M2, Molecular Devices, Sunnyvale, CA, USA).
Western Blot Assay
Treated A549 cells were washed with cold PBS, harvested by resuspending in lysis buffer [25mM HEPES (pH 7.4), 100mM EDTA, 5mM MgCl2, 0.1mM DTT, and a protease inhibitor cocktail], and sonicated to prepare cell lysates. Proteins (20–35 μg) present in the cell lysates were separated by 10%–15% SDSPAGE (polyacrylamide gel electrophoresis) and transferred into nitrocellulose or polyvinylidene difluoride (PVDF) membrane. After incubation with specified concentration of primary antibody in dilution buffer (1% milk with PBS-Tween) and secondary antibody (1:5000), membranes were developed with enhanced chemiluminescence reagents. The following primary antibodies (1:1000) were used for immunoblotting: LC3, p62, and ß-actin (Sigma-Aldrich, St. Louis, MO, USA); cleaved caspase-3, pAMPKα, mTOR (Cell Signaling Technology, Danvers, MA, USA); cleaved caspase-8 (BD pharmingen, USA); DR5 (1:10,000) and DR4 (1:1000) (Abcam). Bands were visualized using a Fusion- FX7 imaging system (Vilber Lourmat, Marne-la-Vallée, France).
Immunocytochemistry
Cells were cultured on glass coverslips, treated with sertraline, washed with PBS, and fixed with 4% paraformaldehyde in PBS at room temperature (RT) for 15 min. Cells were then washed twice with ice cold PBS and incubated at RT for 10 min in PBS containing 0.25% Trion X-100. After incubation, cells were washed three times with PBS. After blocking with 1% BSA in PBST for 30 min, cells were then incubated with primary antibody (anti-p62 and DR5 diluted with 1% BSA in PBST) in a humidified chamber at room temperature for 3 h or at 4 °C overnight. After incubation, solution was decanted, and cells were washed three times with PBS. These cells were then incubated with secondary antibody (diluted with 1% BSA in PBST) in the dark for 2 h at room temperature. Next, the solution was decanted, and cells were washed with PBS three times (5 min per wash). Cells were then incubated with DAPI (4′, 6-diamidino-2-phenylindole) for 10 min followed by PBS rinse. Finally, cells were mounted with fluorescent mounting medium and visualized using a fluorescence microscope (#451203, Nikon ECLIPSE 80i; Nikon Corporation, Tokyo, Japan; magnification, x400).
TEM (Transmission Electron Microscopy) Analysis
TEM samples were analyzed using a Transmission Electron Microscope (JEM-2010, JEOL) installed in the Center for University-Wide Research Facilities (CURF) at Chonbuk National University. After fixing samples in 2% glutaraldehyde (EMS, USA) and 2% paraformaldehyde (EMS, USA) in 0.05 sodium cacodylate buffer (pH7.2) (EMS, USA), specimens were post fixed in 1% osmium tetroxide (EMS, USA) and dehydrated in graded ethanol and propylene oxide (EMS, USA). A549 cells were embedded in Epoxy resin (Embed 812, NMA; Nadic methyl anhydride, DDSA; Dodenyl Succinic Anhydride, DMP-30) (EMS, USA). Ultrathin sections were then cut using an LKB-III ultratome (LEICA, Austria) and stained with 0.5% uranyl acetate (EMS, USA) and lead citrate (EMS, USA). Images were then captured on a Hitachi H7650 electron microscope (Hitachi, Japan) at an accelerating voltage of 100 kV.
RNA Interference
Tested cell lines were transfected with DR5 small interfering RNA (siRNA ID 104279; Ambion, Life Technologies) using Lipofectamine 2000 transfection reagent (Invitrogen) according to the manufacturer’s instructions. At 24-h post transfection, knockdown efficiency was assessed by immunoblotting and cell viability test. Scrambled siRNA (Invitrogen) was used as a negative control.
Statistical Analysis
All data are expressed as means ± standard deviation (SD). Multiple comparisons were performed using one-way analysis of variance (ANOVA) followed by the Tukey–Kramer test. All statistical analyses were performed using GraphPad Prism software. Statistical significance was considered when P value was less than 0.05.
Results
Sertraline induces TRAIL-mediated Apoptosis in Lung Cancer Cell Lines
Effect of sertraline on TRAIL-mediated apoptosis was evaluated by using lung adenocarcinoma cell lines. First, human lung cancer cell lines (A549, HCC-15, and Calu-3) were pretreated with designated concentrations of sertraline for 18 h followed by additional treatment with TRAIL for 2.3 h. Cells were photographed and variations in morphology were examined under a light microscope. Treatment with sertraline or TRAIL alone slightly influenced cell viability. Cells did not show obvious morphological changes compared to the control group (Figures 1-3), indicating that A549, HCC-15, and Calu-3 cells were highly resistant to TRAIL-mediated apoptosis. However, sertraline at varying concentrations with co-treatment of TRAIL meaningfully increased the number of apoptotic cell deaths (Figures 1A, 1B, 2A, 2B and 3A, 3B). Moreover, MTT assay revealed co-treatment with sertraline and TRAIL significantly reduced cell viability and increased the percentage of cells undergoing apoptosis for all tested cells (Figures 1C, 2C, and 3C). When sertraline was used in combination with TRAIL, lactate dehydrogenase (LDH) levels in all cell types were significantly increased, indicating that sertraline combined with TRAIL could significantly induce apoptosis in a dose dependent manner. However, sertraline alone or TRAIL alone did not significantly increase LDH level (Figures 1D, 2D and 3D). These results indicate that sertraline could significantly sensitize TRAIL-mediated apoptosis in TRAIL resistant human lung adenocarcinoma A549, HCC-15, and Calu-3 cells.




Sertraline triggers DR5 up-regulation to induce TRAIL-mediated apoptosis
To explore the molecular mechanism underlying death of A549 cells after combined treatment with sertraline and TRAIL, we investigated death receptors involved in apoptosis (Figure 4). Although TRAIL could bind decoy receptor DcR1, DcR2, and soluble osteoprotegerin, only DR4 and DR5 could produce apoptotic signals through their intracellular death domain. A549 cells were treated with indicated concentrations of sertraline for 18 h. Harvested cell lysates were then subjected to western blot analysis to determine whether DR4 and DR5 were up regulated. Intriguingly, sertraline up-regulated DR5 in a dose-dependent manner in A549 cells but expression of DR4 was unaltered (Figure 4A). Tested cells were pretreated with indicated concentrations of sertraline for 18 h and then exposed to TRAIL for 2h. Intracellular apoptosis regulatory protein cleaved caspase-8 and cleaved caspase-3 were activated in cells after combined treatment with sertraline and TRAIL compared to those in cells treated with sertraline or TRAIL alone (Figure 4B). Furthermore, immunocytochemistry (ICC) showed significant activation of DR5 in sertraline-treated cells compared to that in non-treated cells (Figure 4C). These findings indicate that sertraline could induce TRAIL-mediated apoptosis in lung adenocarcinoma cells via DR5 up-regulation.
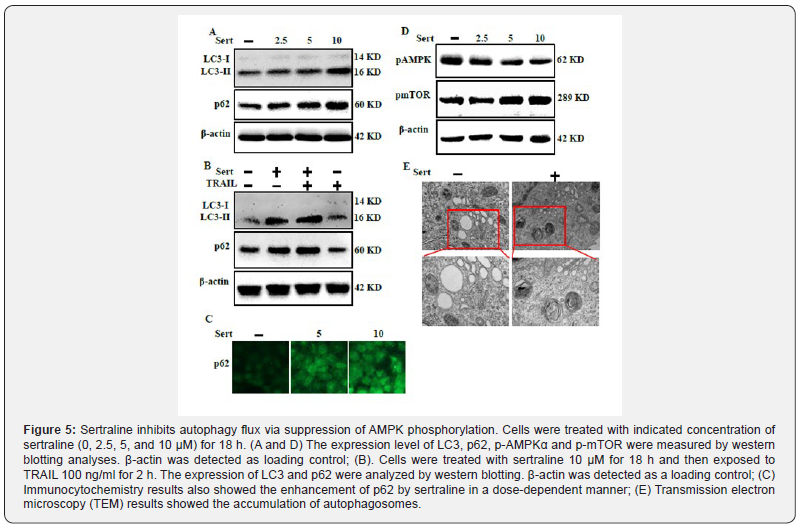
Sertraline inhibits autophagy flux via suppression of AMPK phosphorylation
To investigate the effect of sertraline on autophagy flux, cell lysates were used for western blot to determine changes in LC3- II and p62 (Figure 5). LC3-II formation is a marker of complete autophagosome. p62 is a ubiquitin-binding protein involved in lysosome- and proteasome-dependent protein degradation. Inhibition of autophagy flux results in accumulation of cellular p62 [37]. LC3‑II and p62 expression levels were increased following sertraline treatment, indicating that autophagy flux was inhibited by sertraline (Figure 5A). Treatment with sertraline alone or in combination with TRAIL enhanced LC3-II and p62 levels compared to treatment with TRAIL alone (Figure 5B). ICC results also showed that sertraline enhanced p62 levels in a dose-dependent manner (Figure 5C). AMPK pathway is involved in cellular energy balance by regulating autophagy via suppression of mTOR. Inhibition of AMPK phosphorylation can result in cellular damage and encourage apoptosis by inhibiting autophagy [44-46]. Sertraline inhibited phosphorylation of AMPK mediated energy sensor pathway in a dose-dependent manner, resulting in inhibition of autophagy flux (Figure 5D). Transmission electron microscopy results showed autophagy flux inhibition confirmed by accumulation of highly contained cellular materials containing autophagic vacuoles compared to control (Figure 5E). These findings indicate that inhibition of AMPK phosphorylation by sertraline can inhibits autophagy flux in lung cancer cells.
Sertraline induces enhancement of TRAIL-mediated Apoptosis by Inhibiting Autophagy Flux
Next, we used chloroquine to investigate the effect of sertraline on enhancement of TRAIL-mediated apoptosis in A549 lung adenocarcinoma cells (Figure 6). Chloroquine plays a role as an autophagy inhibitor by blocking acidification of the lysosome [41-42]. Cells were pretreated with chloroquine for 1 h followed by treatment with sertraline at indicated concentrations for 18 h. Cells were then treated with TRAIL protein for an additional 2.3 h. Cells were photographed to investigate their morphological changes using a light microscope. Cell viability was analyzed by crystal violet staining and MTT assay. A549 cells treated with either TRAIL alone or sertraline alone showed slight increase of cell death. However, combined treatment with TRAIL and chloroquine enhanced cell death strongly. Based on cell morphology observation results, combined treatment with TRAIL and chloroquine also enhanced cell death compared to treatment with sertraline alone or TRAIL alone (Figures 6A & 6B). Chloroquine in combination with TRAIL reduced cell viability and significantly increased cell death of A549 lung cancer cells (Figure 6C). LDH assay also showed that chloroquine, sertraline both combined with TRAIL increased apoptotic cell death (Figure 6D). These results indicate that sertraline can enhance TRAIL-mediated apoptosis by inhibiting autophagy flux.

Autophagy flux inhibition causes DR5 up-regulation to enhance TRAIL mediated apoptosis caused by sertraline
We explored the molecular mechanism involved in sertralineinduced enhancement of TRAIL-mediated apoptotic pathway by inhibiting autophagy flux using autophagy inhibitor chloroquine. Autophagy flux inhibition by autophagy inhibitor up-regulated DR5, leading to enhancement of apoptosis (Figure 7). We used two autophagy inhibitors (chloroquine and 3-methyladenine) to treat cells. After treating cells with chloroquine (10μM) or 3-MA for 1 h, cells were then treated with indicated concentration of sertraline for 18 h and then harvested to prepare cell lysates for western blot. Sertraline and a well-known autophagy inhibitor, chloroquine highly increased the p62 and LC3-II levels though 3-MA slightly increased their levels (Figure 7A). Moreover, combined treatment of sertraline with chloroquine and 3-MA up-regulated DR5 level, close to that unregulated by sertraline alone (Figure 7B). To check the activation of intracellular apoptosis indicators cleaved caspase-8 and cleaved caspase-3, cell lysates were collected after pretreatment with chloroquine for 1 h followed by treatment with indicated concentration of sertraline for 18 h and TRAIL protein for an additional 2 h. Inhibition of autophagy by chloroquine and TRAIL also activated cleaved caspase-8 and cleaved caspase-3 (Figure 7C). Taken together, these findings suggest that inhibiting autophagy can up-regulate DR5 and augment sertraline-induced TRAILmediated apoptosis.

Blocking DR5 alters Sertraline-Induced TRAIL mediated apoptosis
Blocking DR5 expression by DR5 siRNA treatment expressively altered cell viability after combined treatment with sertraline and TRAIL (Figure 8). This indicates that DR5 plays a role in sertraline-induced TRAIL-mediated apoptosis. Cells were transfected with DR5 siRNA or a negative control (NC) siRNA for 24 h. Cells were then treated with sertraline for 18 h followed by treatment with TRAIL protein (100 ng/ml) for an additional 2.3 h for cell viability analysis and 2 h for western blotting. We observed that cells transfected with DR5 siRNA showed significantly decreased cell viability that was induced by sertraline and TRAIL co-treatment previously. Additionally, treatment with negative control siRNA resulted in cell viability similar to that observed for cells after sertraline and TRAIL cotreatment (Figures 8A, 8B and 8C). Finally, whole cell lysates were subjected to western blot analysis. Western blot data showed that DR5 activity was inhibited in cells transfected with DR5 siRNA compared to that in non-transfected cells. These experimental findings reveal that up-regulation of DR5 plays an important role in attenuating TRAIL resistance by sertraline. Overall, our findings confirm that inducing TRAIL-mediated apoptosis using sertraline is an attractive therapeutic strategy via AMPK-mediated inhibition of autophagy flux by targeting the TRAIL/DR5 apoptotic pathway

Discussion
Antidepressants are commonly used in cancer patients to release patients’ emotional stress such as depression and dysthymia. Antidepressant drugs such as fluoxetine and sertraline are capable of preventing the effect of stress on tumor progression [46]. It has already been proven that SSRI use might reduce lung cancer risk [47]. Chronic stress decreases antitumor immune response which favors tumor growth [46]. Many studies using animal models have found that behavioral stress encourages faster progression of ovarian cancer [48], pancreatic cancer [49], prostate cancer [50], breast carcinomas [51], and malignant melanomas [52]. TRAIL is considered one of the most promising anti-cancer agents due to its specificity in action by initiating apoptosis in specific cell types and stimulating destruction of cancer cells without affecting normal cells [53-57]. Previous studies have revealed that repetitive administration of TRAIL proteins can adequately weaken tumor growth without affecting normal cells [58,59]. However, a number of cancer cells, including lung cancer cells, are resistant to apoptotic effects of TRAIL [60]. Surprisingly, TRAIL resistance can be overcome by engaging efficient TRAIL-sensitizing pharmacological agents as a combination therapy [61].
We demonstrated that small doses of sertraline in combination with TRAIL could significantly increase the level of apoptotic cell death compared to either treatment alone. These results prove that A549, HCC-15, and Calu-3 cells are TRAIL-resistant lung cancer cells. We also confirmed that sertraline up-regulated DR5, leading to apoptotic cell death when it was combined with TRAIL. These results further clarify that sertraline could attenuate TRAIL resistance and activate the apoptotic caspase cascade (Figures 1-4). Autophagy is a regulated mechanism of the cell, responsible for cell death or survival. It plays a critical role in cell survival by eliminating cytoplasmic proteins and other macromolecules, clearance of damaged organelles, and degradation of misfolded or aggregated proteins [16,17].
Autophagy supports cells with immediate response necessary for recycling indispensable metabolites and fueling the bioenergetic machinery. Many studies have suggested that blocking lysosomal degradation due to inhibition of the autophagy machinery in starved cells can result in faster apoptotic cell death by activating death receptors and apoptotic caspase cascades [39,62-64]. AMPK is the cellular energy sensor that renders survival and proliferation of cells by inducing cytoprotective autophagy via direct inhibition of mTOR. Downregulation of AMPK phosphorylation prompts cellular apoptosis via autophagy inhibition [65,66]. The present findings confirmed that sertraline could increase autophagosome formation indicated by increased LC3-II and trigger defective lysosomal degradation indicated by the accumulation of p62, resulting the inhibition of autophagy flux. This study further revealed that a combined treatment of TRAIL and sertraline or CQ could attenuate cell viability and increase cell death compared to single treatment regimen (Figures 5 & 6). The combined treatment of sertraline and TRAIL induced apoptosis due to inhibition of autophagy flux, which we confirmed by applying autophagy inhibitor chloroquine and 3-MA [67,68] (Figures 6 & 7).
Moreover, we confirmed that inhibition of autophagy by sertraline and specific autophagy inhibitor chloroquine and 3-MA triggered DR5 up-regulation and enhanced TRAIL-mediated caspase-dependent cell death in lung cancer cells (Figure 5). Furthermore, blocking of DR5 expression by using specific DR5 siRNA abrogated cells from TRAIL-mediated apoptosis (Figure 8). Collectively, our results show that sertraline is a potential candidate to attenuate TRAIL resistance and provide an effective combined therapy regimen for lung cancer treatment. Results of this study could be used as basic data for more experiments to select a treatment that is suitable for patients affected by both depression and cancer.
References
- RL Siegel, Miller KD, Jemal Ac (2016) Cancer statistics, 2016. CA: a cancer journal for clinicians 66(1): 7-30.
- S Schelhaas, Held A, Wachsmuth L, Hermann S, Honess DJ, et al. (2016) Gemcitabine Mechanism of Action Confounds Early Assessment of Treatment Response by 3'-Deoxy-3'-[18F]Fluorothymidine in Preclinical Models of Lung Cancer. Cancer Res 76(24): 7096-7105.
- G Garcia, Odaimi M (2017) Systemic Combination Chemotherapy in Elderly Pancreatic Cancer: a Review. J Gastrointest Cancer 48(2): 121-128.
- W Sun, Sanderson PE, Zheng W (2016) Drug combination therapy increases successful drug repositioning. Drug Discov Today 21(7): 1189-1195.
- K Uchibori, Inase N, Araki M, Kamada M, Sato S, et al. (2017) Brigatinib combined with anti-EGFR antibody overcomes osimertinib resistance in EGFR-mutated non-small-cell lung cancer. Nat Commun 8: 14768.
- M Rasheduzzaman, Park SY (2018) Antihypertensive drug-candesartan attenuates TRAIL resistance in human lung cancer via AMPK-mediated inhibition of autophagy flux. Exp Cell Res 368(1): 126-135.
- BB Aggarwal (2003) Signalling pathways of the TNF superfamily: a double-edged sword. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol 3(9): 745-756.
- G Mellier, Huang S, Shenoy K, Pervaiz S (2010) Trailing death in cancer. Mol Aspects Med 31(1): 93-112.
- UM Nazim, Rasheduzzaman M, Lee YJ, Seol DW, et al. (2017) Enhancement of TRAIL-induced apoptosis by 5-fluorouracil requires activating Bax and p53 pathways in TRAIL-resistant lung cancers. Oncotarget 8(11): 18095-18105.
- L Zhang, Fang B (2005) Mechanisms of resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther 12(3): 228-237.
- A Eggert, Grotzer MA, Zuzak TJ, Wiewrodt BR, Ho R, et al. (2001) Resistance to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in neuroblastoma cells correlates with a loss of caspase-8 expression. Cancer Res 61(4): 1314-1319.
- Y Limami, Pinon A, Riaz A, Simon A (2015) TRAIL and targeting cancer cells: between promises and obstacles. TRAIL and targeting cancer cells: between promises and obstacles,/ Cellular and molecular biology (Noisy-le-Grand, France) 61(6): 33-38.
- L O'Leary, van der Sloot AM, Reis CR, Deegan S, Ryan AE, et al. (2016) Decoy receptors block TRAIL sensitivity at a supracellular level: the role of stromal cells in controlling tumour TRAIL sensitivity. Oncogene 35(10): 1261-1270.
- S Wang, El-Deiry WS (2003) TRAIL and apoptosis induction by TNF-family death receptors. Oncogene 22(53): 8628-8633.
- DW Stuckey, Shah K (2013) TRAIL on trial: preclinical advances in cancer therapy,/ Trends in molecular medicine 19(11): 685-694.
- S Wang (2008) The promise of cancer therapeutics targeting the TNF-related apoptosis-inducing ligand and TRAIL receptor pathway. Oncogene 27(48): 6207-6215.
- S Wang (2010) TRAIL: a sword for killing tumors. Current medicinal chemistry 17(29): 3309-3317.
- GS Wu (2009) TRAIL as a target in anti-cancer therapy. Cancer letters 285(1): 1-5.
- CY Jin, Park C, Hwang HJ, Kim GY, Choi BT, et al. (2011) Naringenin up-regulates the expression of death receptor 5 and enhances TRAIL-induced apoptosis in human lung cancer A549 cells. Mol Nutr Food Res 55(2): 300-309.
- X Dai, Zhang J, Arfuso F, Chinnathambi A, Zayed ME, et al. (2015) Targeting TNF-related apoptosis-inducing ligand (TRAIL) receptor by natural products as a potential therapeutic approach for cancer therapy. Exp Biol Med (Maywood) 240(6): 760-773.
- J Ding, Polier G, Kohler R, Giaisi M, Krammer, et al. (2012) Wogonin and related natural flavones overcome tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) protein resistance of tumors by down-regulation of c-FLIP protein and up-regulation of TRAIL receptor 2 expression. J Biol Chem 287(1): 641-649.
- ZG Laoutidis, Mathiak K (2013) Antidepressants in the treatment of depression/depressive symptoms in cancer patients: a systematic review and meta-analysis. BMC psychiatry 13: 140.
- MB Serafin, Bottega A, da Rosa TF, Machado CS, Foletto VS, et al. (2019) Drug Repositioning in Oncology. American journal of therapeutics.
- CT Chou, He S, Jan CR (2007) Paroxetine-induced apoptosis in human osteosarcoma cells: activation of p38 MAP kinase and caspase-3 pathways without involvement of [Ca2+]i elevation. Toxicol Appl Pharmacol 218(3): 265-273.
- M Abdul, Logothetis CJ, Hoosein NM (1995) Growth-inhibitory effects of serotonin uptake inhibitors on human prostate carcinoma cell lines. J Urol 154(1): 247-250.
- A Spanova, Kovaru H, Lisa V, Lukasova E, Rittich B, et al. (1997) Estimation of apoptosis in C6 glioma cells treated with antidepressants. Physiol Res 46(2): 161-164.
- BH Amit, Gil-Ad I, Taler M, Bar M, Zolokov A, et al. (2009) Proapoptotic and chemosensitizing effects of selective serotonin reuptake inhibitors on T cell lymphoma/leukemia (Jurkat) in vitro,/ European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology 19(10): 726-734.
- D Xia, Zhang YT, Xu GP, Yan WW, Pan XR, et al. (2017) Sertraline exerts its antitumor functions through both apoptosis and autophagy pathways in acute myeloid leukemia cells. Leuk Lymphoma 58: 1-10.
- N Mizushima (2007) Autophagy: process and function. Genes & development 21(22): 2861-2873.
- N Mizushima (2009) Physiological functions of autophagy. Curr Top Microbiol Immunol 335: 71-84.
- N Mizushima, Yoshimori T, Levine B (2010) Methods in mammalian autophagy research. Cell 140(3): 313-326.
- E White, DiPaola RS (2009) The double-edged sword of autophagy modulation in cancer. Clin Cancer Res 15(17): 5308-5316.
- Y Kondo, Kanzawa T, Sawaya R, Kondo S (2005) The role of autophagy in cancer development and response to therapy. Nat Rev Cancer 5(9): 726-734.
- Y Kabeya, Mizushima N, Ueno T, Yamamoto A, Kirisako T, et al. (2000) LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 19: 5720-5728.
- I Tanida, Minematsu-Ikeguchi N, Ueno T, Kominami E, et al. (2005) Lysosomal turnover, but not a cellular level, of endogenous LC3 is a marker for autophagy. Autophagy 1(2): 84-91.
- R Gomez-Sanchez, Yakhine-Diop SM, Rodriguez-Arribas M, Bravo-San Pedro JM, Martinez-Chacon G, et al. (2016) mRNA and protein dataset of autophagy markers (LC3 and p62) in several cell lines,/ Data in brief 7: 641-647.
- A Apel, Herr I, Schwarz H, Rodemann HP, Mayer A, et al. (2008) Blocked autophagy sensitizes resistant carcinoma cells to radiation therapy. Cancer research 68(5): 1485-1494.
- JS Carew, Espitia CM, Esquivel JA, 2nd, Mahalingam D, Kelly KR, et al. (2011) Lucanthone is a novel inhibitor of autophagy that induces cathepsin D-mediated apoptosis. J Biol Chem 286(8): 6602-6613.
- P Boya, Gonzalez-Polo RA, Casares N, Perfettini JL, Dessen P, et al. (2005) Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol 25(3): 1025-1040.
- B Poole, Ohkuma S (1981) Effect of weak bases on the intralysosomal pH in mouse peritoneal macrophages. J Cell Biol 90(3): 665-669.
- M Misirkic, Janjetovic K, Vucicevic L, Tovilovic G, Ristic B, et al. (2012) Inhibition of AMPK-dependent autophagy enhances in vitro antiglioma effect of simvastatin. Pharmacol Res 65(1): 111-119.
- BL Heckmann, Yang X, Zhang X, Liu J (2013) The autophagic inhibitor 3-methyladenine potently stimulates PKA-dependent lipolysis in adipocytes. Br J Pharmacol 168(1): 163-171.
- C Zhou, Gu J, Zhang G, Dong D, Yang Q, et al. (2017) AMPK-autophagy inhibition sensitizes icaritin-induced anti-colorectal cancer cell activity,/ Oncotarget 8(9): 14736-14747.
- L Chen, Xu B, Liu L, Luo Y, Zhou H, et al. (2011) Cadmium induction of reactive oxygen species activates the mTOR pathway, leading to neuronal cell death. Free radical biology & medicine 50(5): 624-632.
- BB Kahn, Alquier T, Carling D, Hardie DG (2005) AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell metabolism 1(1): 15-25.
- ME Di Rosso, Sterle HA, Cremaschi GA, Genaro AM (2018) Beneficial Effect of Fluoxetine and Sertraline on Chronic Stress-Induced Tumor Growth and Cell Dissemination in a Mouse Model of Lymphoma: Crucial Role of Antitumor Immunity. Frontiers in immunology 9: 1341.
- S Toh, García Rodríguez LA, Hernández-Díaz (2007) Use of antidepressants and risk of lung cancer. 18(10): 1055-1064.
- PH Thaker, Han LY, Kamat AA, Arevalo JM, Takahashi R, et al. (2006) Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med 12(8): 939-944.
- C Kim-Fuchs, Le CP, Pimentel MA, Shackleford D, Ferrari D, et al. (2014) Chronic stress accelerates pancreatic cancer growth and invasion: a critical role for beta-adrenergic signaling in the pancreatic microenvironment. Brain Behav Immun 40: 40-47.
- S Hassan, Karpova Y, Baiz D, Yancey D, Pullikuth A, et al. (2013) Behavioral stress accelerates prostate cancer development in mice. J Clin Invest 123(2): 874-886.
- EK Sloan, Priceman SJ, Cox BF, Yu S, Pimentel MA, et al. (2010) The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer research 70(18): 7042-7052.
- H Hasegawa, Saiki I (2002) Psychosocial stress augments tumor development through beta-adrenergic activation in mice. Jpn J Cancer Res Gann 93: 729-735.
- FC Kischkel, Lawrence DA, Chuntharapai A, Schow P, Kim KJ, et al. (2000) Apo2L/TRAIL-dependent recruitment of endogenous FADD and caspase-8 to death receptors 4 and 5. Immunity 12(6): 611-620.
- CM Van Geelen, de Vries EG, de Jong S (2004) Lessons from TRAIL-resistance mechanisms in colorectal cancer cells: paving the road to patient-tailored therapy. Drug Resist Updat 7(6): 345-358.
- RK Srivastava (2001) TRAIL/Apo-2L: mechanisms and clinical applications in cancer. Neoplasia 3(6): 535-546.
- S Shankar, Srivastava RK (2004) Enhancement of therapeutic potential of TRAIL by cancer chemotherapy and irradiation: mechanisms and clinical implications,/ Drug resistance updates: reviews and commentaries in antimicrobial and anticancer chemotherapy 7(2): 139-156.
- H LeBlanc, Lawrence D, Varfolomeev E, Totpal K, Morlan J, et al. (2002) Tumor-cell resistance to death receptor--induced apoptosis through mutational inactivation of the proapoptotic Bcl-2 homolog Bax. Nature medicine 8(3): 274-281.
- AC Bellail, Qi L, Mulligan P, Chhabra V, Hao C (2009) TRAIL agonists on clinical trials for cancer therapy: the promises and the challenges. Reviews on recent clinical trials 4(1): 34-41.
- H Walczak, Miller RE, Ariail K, Gliniak B, Griffith TS, et al. (1999) Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med 5(2): 157-163.
- DJ Klionsky (2007) Autophagy: from phenomenology to molecular understanding in less than a decade Nat Rev Mol Cell Biol 8(11): 931-937.
- S Shimizu, Kanaseki T, Mizushima N, Mizuta T, Arakawa-Kobayashi S, et al. (2004) Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nature cell biology 6(12): 1221-1228.
- EJ Park, Min KJ, Choi KS, Kubatka P, Kruzliak P, et al. (2016) Chloroquine enhances TRAIL-mediated apoptosis through up-regulation of DR5 by stabilization of mRNA and protein in cancer cells. Sci Rep 6: 22921.
- RA Gonzalez-Polo, Boya P, Pauleau AL, Jalil A, Larochette N, et al. (2005) The apoptosis/autophagy paradox: autophagic vacuolization before apoptotic death. J Cell Sci 118: 3091-3102.
- T Wiedmer, Blank A, Pantasis S, Normand L, Bill R, et al. (2017) Autophagy Inhibition Improves Sunitinib Efficacy in Pancreatic Neuroendocrine Tumors via a Lysosome-dependent Mechanism,/ Molecular cancer therapeutics 16(11): 2502-2515.
- MH Liu, Lin XL, Guo DM, Zhang Y, Yuan C, et al. (2016) Resveratrol protects cardiomyocytes from doxorubicin-induced apoptosis through the AMPK/P53 pathway. Mol Med Rep 13(2): 1281-1286.
- X Liu, Chhipa RR, Nakano I, Dasgupta B (2014) The AMPK inhibitor compound C is a potent AMPK-independent antiglioma agent. Molecular cancer therapeutics 13: 596-605.
- X Hu, Shi S, Wang H, Yu X, Wang Q, et al. (2017) Blocking autophagy improves the anti-tumor activity of afatinib in lung adenocarcinoma with activating EGFR mutations in vitro and in vivo. Scientific reports 7: 4559.
- YC Lin, Lin JF, Wen SI, Yang SC, Tsai TF, et al. (2017) Chloroquine and hydroxychloroquine inhibit bladder cancer cell growth by targeting basal autophagy and enhancing apoptosis,/ The Kaohsiung journal of medical sciences 33(5): 215-223.






























