Ipsilateral Pleura Radiotherapy Combined Intrapleural with Intravenous Chemotherapy for Patients with NSCLC Presenting Malignant Pleural Effusion
Md Tofiz Uddin*, Yanling Bai and Yu Lijuan
Department of Medical Physics, Radiation Oncology, and Department of Imaging and Nuclear Medicine, Harbin Medical University Cancer Hospital, China
Submission: March 12, 2019: Published: April 12, 2019
*Corresponding Address: Md Tofiz Uddin, Department of Medical Physics, Radiation Oncology, and Department of Imaging and Nuclear Medicine, Harbin Medical University Cancer Hospital, Harbin 150081, China
How to cite this article: Md Tofiz U, Yanling B, Y Lijuan. Ipsilateral Pleura Radiotherapy Combined Intrapleural with Intravenous Chemotherapy for Patients with NSCLC Presenting Malignant Pleural Effusion. Canc Therapy & Oncol Int J. 2019; 13(3): 555862. DOI:10.19080/CTOIJ.2019.13.555862
Abstract
Purpose: Patients with non-small cell lung cancer (NSCLC) and malignant pleural effusion (MPE) are difficult to manage clinically. In this study, we designed a protocol of combined ipsilateral pleura radiotherapy with intrapleural(i.p.) and intravenous(i.v.) chemotherapy to enhance local as well as systemic control of the disease.
Method: From January 2006 to January 2011, 31 patients with NSCLC and MPE were eligible for the study. After adequate drainage, patients received chemotherapy (cisplatin 60mg/m2 i.p. on day 1, paclitaxel 125mg/m2 and cisplatin 75mg/m2 on days 3 and 31 i.v.), and after first cycle chemotherapy followed by forward IMRT. Radiation targets included primary tumor, mediastinal, lymphatic drainage area, the ipsilateral pleura and 1/2 pleural effusion. The radiation dose is 190cGy / fraction. After 6080cGy/32 fractions delivered, residues tumours were given with boost irradiation 570~950 cGy /3~5 fractions.
Result: Overall response of pleural effusion was 90.3% with 38.7% complete remission, 51.6% with partial remission, 6.5% stable disease, and 3.2 % progressive disease. The median failure-free and overall survival was 13 and18 months, respectively. 1- and 2-years overall survival was 77.3% and 19.9%. Grade 1, 2, 3 and 4 of lung and esophagus acute radiation-induced toxicity was 58.1%,19.4%,3.2% and 0% and 48.4%9.7%, 3.2% and 0%. The rate of grade 1, 2, 3 and 4 with hematologic toxicity was 38.5%, 23.1%, 3.8%, and 0%.
Conclusion: Ipsilateral pleura radiotherapy combined with intrapleural and intravenous Chemotherapy for patients with NSCLC presenting malignant pleural effusion is feasible, effective, and provides an alternative treatment modality for them.
Keywords: Malignant pleural effusion; Chemotherapy; Pleura radiotherapy; Non-small cell lung cancer
Introduction
Lung cancer is the leading cause of malignant pleural effusion (MPE) [1-5]. Approximately 15% of lung cancer patients have pleural effusion at the time of initial diagnosis and 50% develop pleural effusion later in their causes [3,5,6]. Patients with pleural effusion have life short expectancy and are difficult to manage clinically [3,4]. A more definitive management strategy is often undertaken with several approaches available to physician or surgeon. These options include repeat thoracentesis, tube thoracostomy with chemical pleurodesis, placement of an indwelling, cuffed, tunned pleural catheter (ICTPC) with or without pleurodesis or medical pleuroscopy or video-assisted theracoscopic surgery (VATS) with pleurodesis [7-16]. The most common therapy for these NSCLC patients with MPE is a tube thoracostomy drainage and intrapleural instillation of a chemical sclerosing agent with or without subsequent systemic chemotherapy [17-19]. Although current mode of therapy can occasionally alleviate the symptoms, the relapse rate is as high as 50% [20].
MPE in patients with NSCLC portents a poor prognosis, with a median survival of 3 ~5 months [21]. On occasion, MPE can be managed by treating the underlying malignancy with contemporary antineoplastic agents and/ or radiotherapy. Unfortunately, in majority of cases, the MPE either dose not resolve or recurs after initial drainage [16]. Only one study showed that pulmonary irradiation combined with intrapleural and intravenous chemotherapy could prolong the patient’s overall survival (OS) [22]. The patients with other distant metastases did not enrolled in the study except for bone metastases [22]. Because the major causes of death for patients with NSCLC and MPE include progression of pulmonary disease, pleural involvement, and organ failure resulting from distant metastases, a combined-modality approach that can effectively control pulmonary, pleural metastatic lesions appears necessary to improve the outcome of this group patients, we therefore designed and evaluated the treatment protocol consisting of ipsilateral pleura and pulmonary irradiation, i.p and i.v chemotherapy to treat patients with NSCLC presenting with symptomatic MEP.
Patients and Methods
Patient Eligibility
Eligibility criteria included histopathological NSCLC and symptomatic MPE at the time of initial diagnosis, Karnofsky performance status>70, liver and kidney function were normal, and expected survival >3 months. From January 2006 to January 2011, 31 patients were eligible for this study. Median age was 57(33-70) years. Of the 31 cases, 15 had distant metastasis, which included bone, brain, liver and adrenal. The clinical characteristics showed in Table 1. Initial evaluation consisted of a complete history and physical examination, serum chemistry, a complete blood cell count, and serum tumor marker (CEA). A computed tomographic (CT) scan of the chest to the level of the adrenal glands, an abdominal sonographic examination, a radionuclide bone scan, and magnetic resonace imaging (MRI) of the brain were also performed for comprehensive staging. Effusion that occupied more than three-fourths of the hemithorax was classified as ‘ massive’, between one- half and three-fourths as ‘large .’and between one-fourths and one-half as moderate‘moderate’and less than one-fourths as ‘small’ All eligible patients had a Karnofsky performance status of 70-100 and bidimensional measurable disease, but none had had prior chemo- radiotherapy or prior pleurodesis. Before treatment, all patients signed an informed consent form (Table 1).
The treatment response of malignant effusion was evaluated according to the following criteria:
i. Complete response (CR), no fluid reaccumulation and patients were free of symptoms for at least 4 weeks determined by chest CT scan;
ii. Partial response (PR), effusion decrease more than 50% of the original effusion volume, patients were asymptomatic and no need for thoracentesis for symptom relief within 4 weeks after treatment;
iii. Stable disease (SD), effusion decrease less than 50% of the original volume;
iv. Progression disease (PD), recurrence of effusion greater than 50% of the original volume, patients were symptomatic and need for thoracentesis to relieve symptoms with 4 weeks of treatment. The toxicities of the therapy were evaluated according to Radiation Oncology Therapy Group (RTOG) toxicity criteria.
Intrapleural and Intravenous Chemotherapy
First, ultrasound was used to confirm puncture point. Under local anaesthesia a puncture needle was inserted. 800~1200ml pleural effusion was drained, and then 60mg cisplatin in 40ml normal saline injected into the pleural cavity. After 2 days of the thoracentesis, paclitaxel 125mg/m2 and cisplatin75mg/m2 were administered i.v. and followed by radiotherapy. After 3800cGy /20 factions were delivered, the second cycle chemotherapy was given, and then the radiotherapy continued.
Radiotherapy
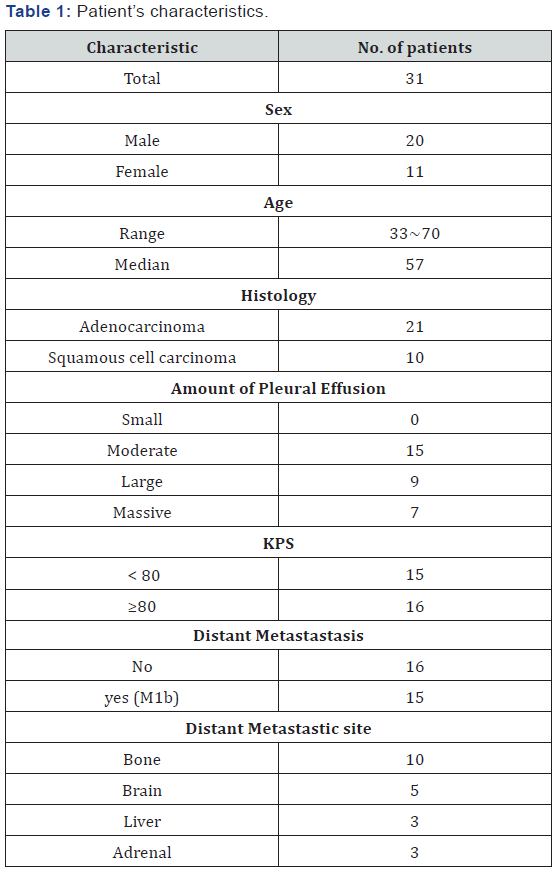
Patients were positioned supine on the table with their arms above their head. MedTech body frame and thermoplastic body mask were utilized. CT simulation was performed for the patients using PHILIPS Brilliance Big Bore with 5 mm slice. Radiation targets included primary tumor, mediastinal, bilateral supraclavicular area, the ipsilateral pleura and 1/2 pleural effusion. The CT images were transferred to Elekta Precise 2.03 planning system to perform the forward treatment planning (FTP), which was previously described by Bai Yanling et al. [23]. The concept and method of FTP as follow: The geometric centre of the target was defined as the plan isocentre. Revolving tangential beams were designed along the chest wall to avoid direct irradiation of the normal lung tissue. The beam weights, directions, shape of MLC and other parameters were adjusted according to the dose distribution until the results meet the clinical needs. The average number of fields was 23 (18 to33) The evaluation results of FTP for all patients as follow: 90% isodose line included more than 90% GTV volume. 70% isodose line included more than 90% CTV volume under the premise of reducing the lungs dose.
For ipsilateral lung of all patients, the average volume included in 50% isodose line was 29% (20~35%), and in 30% isodose line was 48% (33~65%). For the contralateral lung, the volume included in 50% isodose line were less than 3%, and in 30% isodose line were less than 10%. The maximum spinal isodose was kept < 80%. The average volume of heart in primary tumor of right lung included in 50% isodose curve was 21% (15~25%), and that in the left lung was 45% (31~68%) (Figure 1). Radiation dose(90% isodose curve)is 190cGy / fraction, 5 times / week. After 3800cGy/20fractions,second CT simulation scan was performed. Radiation target included primary, lymphatic and pleural lesions, and 1/2 pleural effusion. After 2280cGy/12 fractions was delivered, third CT simulation scan was done, and the residual tumours were given with boost irradiation 570~950 cGy /3~5 fractions. The total radiation dose was 6650~7030cGy /35~37 fractions. In the same period, the distant metastatic lesions were also given radiotherapy.
Follow up
The survival time of all patients was measured from the day of registration until the date of death, living patients were censored on the date of the last follow-up examination. Failure free survival (i, e. death or disease progression). Media follow-up time 18(range, 6-54) months and the follow-up rate was 100%.
Statistical Analysis
SPSS version 17.0 (SPSS, Chicago, IL) was used for statistical analysis. The method of Kaplan-Meier was used to describe survival and failure-free survival. The differences were compared with log-rank test in the univariate analysis. P values less than or equal to 0.05 were significant.
Results
During the chemoradiation, 8 patients had still dyspnoea, who given the second aspiration of pleural fluid, and the 3/8 were given the third aspiration of pleural fluid. RTOG grade 1,2,3 and 4 of lung and esophagus acute radiation-induced toxicity was 58.1%, 19.4%, 3.2% and 0% and 48.4%9.7%, 3.2% and 0%. The rate of grade 1, 2, 3 and 4 with hematologic toxicity was 38.5%, 23.1%, 3.8%.and 0%. In general, the lung and hematologic toxicities were mild. Only one patient experienced RTOG grade 3 pneumonitis and two had grade 3 leukopenia (Table 2). Overall response of pleural effusion was 90.3% with 38.7% complete remission, 51.6% with partial remission, 6.5% stable disease, and 3.2 % progressive disease. Only one patient experienced reaccumulation of pleural effusion. The median failure-free and overall survival was 13 and18 months, respectively. The 6, 12- and 24-month OS was 90.3%, 77.3% and 19.9%, respectively. Univariate analysis showed that Karnofsky performance status (KPS), thoracic fluid volume, T and N staging, and radiation dose were prognostic factors (p=0.001, 0.000, 0.000 and 0.000) (Table 3). Median survival time of the patients with KPS ≥80, moderate pleural effusion, N1, T1, and radiation dose ≥66.5Gy was 20, 20, 37.5, 23, and 20 months respectively.
Discussion
MPE is a common complication in patients with NSCLC, which is difficult to manage clinically. The most common therapy for these patients is a tube thoracostomy drainage and intrapleural instillation of a chemical sclerosing agent with or without subsequent systemic chemotherapy. MPE in patients with NSCLC portents a poor prognosis, with a median survival of 3 ~5 months, which estimated 1, 2-year survival 12.6% ~24.8% and 5.4%~11.3% [22]. Most patients with NSCLC and MPE die of intrapulmonary progression, intractable intrapleural effusion, systemic metastasis, or a combination of these. We therefore injected cisplatin into the pleural cavity to enhance killing tumor cells seeding the pleurae. Paclitaxel, an effective firstline agent for NSCLC, was given intravenously two cycles during the chemoradiation to control systemic disease. Ipsilateral pleura radiotherapy was introduced to enhance local control of intrapulmonary diseases, pleural involvement, tumor cells in pleural fluid. The overall response rate in measurable lesions to this combined modality treatment was 87.1%, and the control rate of pleural effusion was 90.3% through the whole clinical course. The median failure-free and overall survival was 13 and 18 months. 1,2-year survival rate was 70.3%, and 19.9%, which were higher than the results reported by Wu-Chu Su et al. [22]. Wu-Chu Su`s study only included patients with bone metastasis. Before radiotherapy, 4 cycles intrapleural and intravenous chemotherapy were given, and one month after radiotherapy 7020cGy/39F, 3-6 cycle chemotherapy were administered i.v.
The median failure-free and overall survival was 8 and 16 months, and 1-year survival rate was 63.0%. 22 The clinical results of the multiple cycle chemotherapy (4 times i.p. and 6-9 cycles i.v.) combined with pulmonary irradiation was not as good as that of less cycle chemotherapy (1-time i.p and 2 cycles i.v.) combined with ipsilateral pleurae radiotherapy in our study. The main difference between the two studies was that ipsilateral pleurae radiotherapy was introduced in our study and did not in Su`s study. MPE occurs mostly because of impaired lymphatic drainage, anywhere from tumor occlusion of parietal pleura stomata to enlarged mediastinal lymph nodes. 19 In patients with lung cancer, MPEs are usually caused by pulmonary artery invasion and embolization of tumor cells to the visceral pleura or direct invasion from peripheral tumours [24]. So, the ipsilateral pleurae radiotherapy was an effective method for patients with NSCLC and MPE.
Morgensztern et al. [21] study showed a significant prognostic role for MPE, which was associated with a decreased median OS from 5 months to 3 months in patients with stage M1b disease [21]. Our study found that there was not difference between OS of patients with distant(M1b) and no distant metastases (20 months versus 18 months) (Table 3). This mainly was because the distant metastatic lesions in our study were given radiotherapy. Univariate analysis in our study also found that predictors for poor outcomes were KPS<80, massive amount of pleural effusion, advanced T and N stage, and lower radiation dose (Table 3).
Radiotherapy was rarely used for treating patients with NSCLC and MPE, mainly because the conventional radiation techniques increase radiation damage of normal lung tissue [25]. Thorax cancer of patients who received radical radiotherapy approximately 13% to 37% occurred radiation pneumonitis( RP) [26]. In our study, the forward treatment planning (FTP) and boost radiation technique were used for the patients with NSCLC and MPE, which not only increased tumor radiation dose, but also lung radiation side effects were acceptable. Of the 31 patients, 6(19.4%) and 1(3.2%) experienced grade 2 and 3 acute pulmonary toxicity respectively. In conclusion ipsilateral pleura radiotherapy combined with intrapleural and intravenous Chemotherapy for patients with NSCLC presenting malignant pleural effusion is feasible, effective, and provides an alternative treatment modality for them.
Author Disclosures of Potential Conflicts of Interest
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked a “U” are those for which no compensation was received; those relationship marked a “C” were compensated, or for more information about ASCO’S conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosure of Potential Conflict of Interest section in Information contributors.
Author Contributions
Conception and design: Md Tofiz Uddin
Collection and assembly of data: Md Tofiz Uddin ad Yanling Bai
Manuscript and writing: all authors
Final approval of manuscript: all authors
This work was supported by project research (81671771) from the Natural Science foundation China. Author disclosures of potential conflicts of interest and author contributions are found at the end of this article.
References
- Johnston WW (1985) The malignant pleural effusion: a review of cytopathologic diagnoses of 584 specimens from 472 consecutive patients. Cancer 56: 905-909.
- Chernow B, Shala SA (1973) Carcinoma involvement of the pleura: analysis of 96 patients. Am J Med 63(5): 695-702.
- Hott JW, Ma-PE (1995) Malignant pleural effusion. Semin Respir Crit Care Med 16: 33-39.
- Iran DR, Unerwood RD, Johson EH (1987) MPEs- a clinical cytopathologic study. Arch Inter Med 147: 1133-1136.
- Chens, Hossain MS (1966) Primary carcinoma of lung: a review of 417 histologically proved cases. Dis Chest 49(1): 67-74.
- Perng RP, Chen YM, Wu MF, Chou KC, Lin WC, et al. (1998) PhaseⅡ trial intrapleural palitaxel injection for non-small cell lung cancer with malignant pleural effusion. Respiratory Medicine 92(3): 473-479.
- DK Muduly, SVS Deo, TS Subi, AA Kallianpur, NK Shukla (2011) Aa update the management of malignant pleural effusion. Indian J of palliative care 17(2): 98-103.
- Jones PW, Moyer JP, Rogers JT, Rodriguez RM, Lee YC, (2003) Ultrasound guided thoracentesis: is it a safer method? J Chest 123(2): 418-423.
- de Campos JR, Vargas FS, de Campos Werebe E, Cardoso P, Teixeira LR, et al. (2001) Thoracoscopy talc poudrage: a 15-year experience. J Chest 119(3): 801-806.
- Tremblay A, Michaud G (2006) Single-center experience with 250 tunnelled pleural catheter insertions for malignant pleural effusion. Chest 129(2): 362-368.
- Tremblay A, Mason C, Michaud G (2007) Use of tunnelled catheters for malignant pleural effusions in patients fit for pleurodesis. Eur Respir J 30: 759-762.
- Trotter D, Aly A, Siu L, Knight S (2005) Video-assisted thoracoscopic [VATS] pleurodesis for malignant effusion: an Australian teaching hospital’s experience J. Heart Lung Circ 14(2): 93–97.
- Marrazzo A, Noto A, Casà L, Taormina P, Lo Gerfo D, David M, et al. (2005) Video-thoracoscopic surgical pleurodesis in the management of malignant pleural effusion: the importance of an early intervention. J Pain Symptom Manage 30(1): 75–79.
- Bazerbashi S, Villaquiran J, Awan MY, Unsworth-White MJ, Rahamim J, et al. (2009) Ambalatory intercostal drainage for the mangement of malignant pleural effusion: a single experience. Ann Surg Oncol 16(12): 3482-3487.
- Van Meter ME1, McKee KY, Kohlwes RJ (2010) Efficacy and safety of tunneled catheters in adults with malignant pleural effusion:A systematic review. J Gen Interm Med 26(1): 70-76.
- Haas AR, Sterman Dani DH, Musani AI (2007) Malignant pleural effusions: management options with consideration of coding, biling, and a decision approach Chest 132(3): 1036-1041.
- Seto T, Ushijima S, Yamamoto H, Ito K, Araki J, et al. (2006) Intrapleural hypotonic cisplatin treatment for malignant pleural effusion in 80 patients with non-small-cell lung cancer J. British Journal of Cancer 95(6): 717-721.
- Yoshida K, Sugiura T, Takifuji N, Kawahara M, Matsui K, et al. (2007) Randomized phase II trial of three intrapleural therapy regimens for the management of malignant pleural effusion in previously untreated non-small cell lung cancer. J Lung Cancer 58(3): 362-368.
- Imran Zahid, Tom Routledge, Andren Billé, Marco Scarci (2011) What is the best treatment for malignant pleural effusion? Interactive Cardiovascular and Thoracic Surgery (12): 818-823.
- Nan Du, Xiaosong Li, Zhao H, Fan Z, Feng Li, et al. (2013) Intrapleural combination therapy with bevacizumab and cisplatin for no-small cell lung cancer- mediated malignat pleural effusion. Oncology Reports 29(6): 2332-2340.
- Morgensztern D, Waqar S, Subramanian J, Trinkaus K, Govindan R (2012) Pronnostic impact of malignant pleural effusion at presentation in patients with no-smal-celll lung cancer J Thorac Oncol 7(10): 1485- 1489.
- Su WC, Lai WW, Chen HH, Hsiue TR, Chen CW, et al. (2003) Conbined intrapleural and intravennous, and pulmonary irradiation, for treatment of patients with lung cancer presenting with malignant pleural effusion. Oncology 64(1): 18-24.
- Bai Yan-ling, Yun Wen-kang, Hu Hong-tao, Liu Chengji (2006) Forward IMRT planning of nasopharyngeal carcinoma by field aperture shape optimazing based beam direction. Cin J Radiat Oncol 15(6): 489-495.
- Lynch TJ Jr (1993) Management of malignant pleural effusion. Chest 103(4 Suppl): 385S-389S.
- Schid SE, Stella PJ, Gever SM, Bonner JA, McGinnis WL, et al. (2003) The outcome of combined modality therapy for stage Ⅲ non-small cell lung cancer inthe ederly. J Clin Oncol 21(17): 3201-3206.
- Clade L, Perol O, Ginestet C, Falchero L, Arpin D, et al. (2004) A prospective on radiation pneimonitis following conformal radiation therapy in non-small cell lung cancer: clinical and dosimetric factors analysis. J Radiother Oncol 71(2): 175-181.






























