Evaluation of Target Volume Determination for Single Session Stereotactic Radiosurgery (SRS) of Brain Metastases
Selcuk Demiral*, Omer Sager, Ferrat Dincoglan, Bora Uysal, Hakan Gamsiz, Bahar Dirican and Murat Beyzadeoglu
Department of Radiation Oncology; University of Health Sciences, Gulhane Medical Faculty, Ankara, Turkey
Submission: December 21, 2018;Published: December 13, 2018
*Corresponding Address: Selcuk Demiral, University of Health Sciences, Gulhane Medical Faculty, Department of Radiation Oncology, Gn.Tevfik Saglam Cad. 06018, Etlik, Kecioren, Ankara, Turkey
How to cite this article: Selcuk D, Omer S, Ferrat D, Bora U, Hakan G, et al. Evaluation of Target Volume Determination for Single Session Stereotactic Radiosurgery (SRS) of Brain Metastases. Canc Therapy & Oncol Int J. 2018; 12(5): 555848. DOI:10.19080/CTOIJ.2018.12.555848
Abstract
Aims and background: Brain metastases comprise the most common intracranial neoplasms in adults. When determining the treatment modality for brain metastases, expected survival duration and quality of life aspects should be taken into consideration. Stereotactic Radiosurgery (SRS) has emerged as a viable radiotherapeutic modality for management of brain metastases. Target volume definition is a critical component of SRS of brain metastases due to typically steep dose gradients around the treatment volume. Incorporation of multimodality imaging may improve determination of target volume for radiosurgery. In this context, we evaluated the utility of multimodality imaging for target volume determination for single session SRS of brain metastases in this study.
Materials and Methods: We retrospectively identified 18 patients receiving single session SRS for brain metastases at our department. Target volumes for SRS were determined based on either CT simulation images only or by fusion of T1 gadolinium-enhanced volumetric Magnetic Resonance (MR) images acquired the day before SRS using 1 mm slice thickness. The 2 target volumes acquired from CT-only based imaging and CT-MR fusion based imaging for each patient were comparatively assessed.
Results: A total of 18 patients receiving single session SRS for brain metastases at our department were assessed for target volume determination based on CT-only imaging and CT-MR fusion based imaging. Mean target volume based on CT-only imaging and CT-MR fusion based imaging, and consensus decision of all treating radiation oncologists was 5.2 cc (range: 0.8-13.1 cc), 4.8 cc (range: 0.9-12.9 cc), and 4.9 cc (range: 0.9-13 cc), respectively.
Conclusion: Our study revealed that determination of target volumes for brain metastasis radiosurgery may be improved by use of CT-MR fusion based imaging. Clearly, further studies are warranted to investigate the utility of multimodality imaging for target volume determination for SRS of brain metastases.
Keywords: Brain Metastasis; Stereotactic Radiosurgery (SRS); Target Volume
Abbreviation: SRS: Stereotactic Radiosurgery; WBRT: Whole Brain Radiotherapy; QOL: Quality of Life; RPA: Recursive Partitioning Analysis; KPS: Karnofsky Performance Status; SRS: Stereotactic Radiosurgery; OAR: Organ-At-Risk
Introduction
Brain metastases comprise the most common intracranial neoplasms in adults. 20%-40% of patients having systemic cancer experience brain metastasis at some point [1-4]. Longer survival with more effective systemic treatments and improvements in neuroimaging led to more frequent detection of brain metastases in recent years. Primaries for brain metastases include lung cancer in 40%-60% of the patients, breast cancer in 15%-20% of the patients, melanoma in 10%-20% of the patients, colorectal Ca in 5%-10% of the patients, renal cell Ca in 5%-10% of the patients and unknown in 15% of the patients [5,6]. The prognosis of these patients with brain metastasis is poor. Median survival for patients with symptomatic brain metastasis is about 4 weeks if untreated, and about 3-6 months if conventional Whole Brain Radiotherapy (WBRT) is given [7]. When determining the treatment modality for brain metastases, expected survival duration and quality of life aspects should be taken into consideration.
Symptoms of brain metatasis are various depending on size, number and location which may greatly affect the patients’ quality of life (QOL). Long treatment and hospitalization times may worsen their QOL. Recursive Partitioning Analysis (RPA) was developed to determine prognostic factors for patients with brain metastasis and classifies patients into 3 prognostic groups by using pretreatment factors including Karnofsky Performance Status (KPS), age, status of the primary tumor (controlled vs. uncontrolled) and extracranial metastasis [8]. Median survival was reported to be 7.1 months for RPA class I patients, 4.2 months for RPA class II patients, and 2.3 months for RPA class III patients [8]. Treatment options may vary for different RPA classes.
Multimodality management may be utilized for treatment of brain metastases using combinations of surgery, WBRT, Stereotactic Radiosurgery (SRS), and systemic agents. SRS has emerged as a viable radiotherapeutic modality for management of various benign and malign conditions throughout the human body [9-27]. Target volume definition is a critical component of SRS of brain metastases due to typically steep dose gradients around the treatment volume. Incorporation of multimodality imaging may improve determination of target volume for radiosurgery. In this context, we evaluated the utility of multimodality imaging for target volume determination for single session SRS of brain metastases in this study.
Materials and Methods
We retrospectively identified 18 patients receiving single session SRS for brain metastases at our department. An informed consent was taken from every patient before treatment. Treatment with SRS was decided by a multidisciplinary team including experts on radiation oncology, neurosurgery, and neuroradiology.
On the day of treatment, a stereotactic frame was affixed with 4 pins to the patients’ skull under local anesthesia, and contrastenhanced planning Computed Tomography (CT) images were acquired at CT simulator (GE Lightspeed RT, GE Healthcare, Chalfont St. Giles, UK) using a slice thickness of 1.25 mm. Image data sets were sent to the contouring workstation (SimMD, GE, UK) for delineation of target volume and critical structures typically including the brainstem, optic nerves, chiasm, and other relevant critical structures in close vicinity of the target. For the purpose of this study, target volumes were determined based on either CT simulation images only or by fusion of T1 gadoliniumenhanced volumetric Magnetic Resonance (MR) images acquired the day before SRS using 1 mm slice thickness. The 2 target volumes acquired from CT-only based imaging and CT-MR fusion based imaging for each patient were comparatively assessed. For treatment and comparison purposes, determination of ground truth target volume for each patient was decided by consensus and collaboration of the treating radiation oncologists. ERGO ++ (CMS, Elekta, UK) radiosurgery planning system and Synergy (Elekta, UK) Linear Accelerator (LINAC) with 3 mm thickness head-on micro multileaf collimator (micro-MLC) was used for treatment planning and delivery, respectively.
A single 360-degree arc, double 360-degree arcs, four 90-degree arcs or five 180-degree arcs were selected in radiosurgery treatment planning for optimal sparing of critical structures surrounding the target. Windows and levels of the planning CT simulation images were adjusted so as to achieve improved visualization of the target and critical structures. Coronal and sagittal images were used in combination with axial images to improve target and organ-at-risk (OAR) delineation accuracy. Arc Modulation Optimization Algorithm (AMOA) was utilized for providing improved target coverage whilst sparing neighbouring critical structures. Median dose for SRS was 20 Gy (range: 18-24 Gy) prescribed to the 85%-95% isodose line encompassing the target volume. kV-CBCT (kilovoltage Cone Beam CT) was used for verification of isocenters along with the XVI (X-ray Volumetric Imaging, Elekta, UK) system for setup verification. Intravenous dexamethasone with H2- antihistamines was used immediately after SRS for all patients.
Results
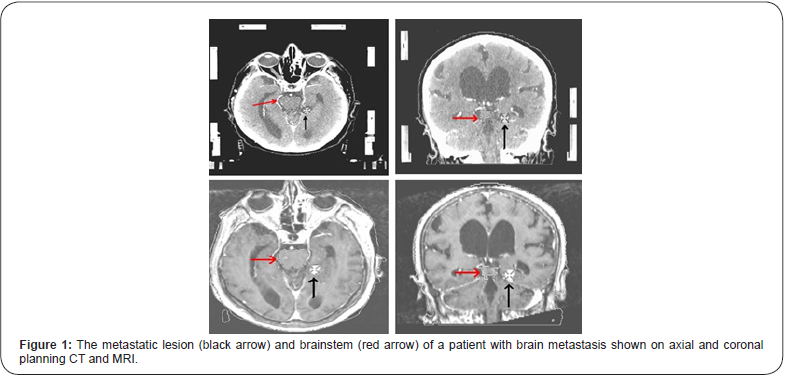
A total of 18 patients receiving single session SRS for brain metastases at our department were assessed for target volume determination based on CT-only imaging and CT-MR fusion based imaging. Mean target volume based on CT-only imaging and CT-MR fusion based imaging, and consensus decision of all treating radiation oncologists was 5.2 cc (range: 0.8-13.1 cc), 4.8 cc (range: 0.9-12.9 cc), and 4.9 cc (range: 0.9-13 cc), respectively. Target determination based on CT-MR fusion based imaging was identical to consensus decision of all treating radiation oncologists in the great majority of patients.
Figure 1 shows the metastatic lesion (black arrow) and brainstem (red arrow) of a patient with brain metastasis on axial and coronal planning CT and MR images.
Discussion
Brain metastasis is a common complication of systemic cancer. There is not a widely accepted consensus for optimal combined-modality treatment of patients with brain metastases due to the difficulty in defining the role of combination of modalities including WBRT, surgery and SRS in inhomogeneous patient populations among studies. The pseudospherical shape of brain metastases makes these lesions optimal targets for radiosurgery. The frequent location of the metastatic lesions at the junction of gray and white matter allows the application of higher single doses since this region is relatively noneloquent. MRI, which is increasingly being used for neuroimaging, allows the detection of smaller lesions (< 3 cm) suitable for SRS.
There is still room for improvement to achieve optimal therapeutic outcomes for patients with benign and malignant brain tumors using various combinations of surgery, radiation therapy, systemic agents and immunotherapy [28-30]. In the context of brain metastases management, our study adds to the compiling body of evidence indicating improved target definition for brain metastasis radiosurgery with incorporation of MRI into SRS treatment planning [31-34].
In conclusion, our study revealed that determination of target volumes for brain metastasis radiosurgery may be improved by use of CT-MR fusion based imaging, and consensus decision of all treating radiation oncologists was identical to target volume determination with CT-MR fusion based imaging in the great majority of patients. Clearly, further studies are warranted to investigate the utility of multimodality imaging for target volume determination for SRS of brain metastases.
Conflict of Interest
There are no conflicts of interest and no acknowledgements.
References
- Nussbaum ES, Djalilian HR, Cho KH, Hall WA (1996) Brain metastases. Histology, multiplicity, surgery, and survival. Cancer 78(8): 1781-1788.
- Posner JB (1992) Management of brain metastases. Rev Neurol 148(6- 7): 477-487.
- Walker AE, Robins M, Weinfeld FD (1985) Epidemiology of brain tumours: the national survey of intracranial neoplasms. Neurology 35: 219-226.
- Zimm S, Wampler GL, Stablein D, Hazra T, Young HF (1981) Intracerebral metastases in solid-tumor patients: natural history and results of treatment. Cancer 48(2): 384-394.
- Kihlstrom L, Karlsson B, Lindquist C (1993) Gamma knife surgery for cerebral metastases. Implications for survival based on 16 years experience. Stereotact Funct Neurosurg 61 Suppl 1: 45-50.
- Posner JB (1974) Diagnosis and treatment of metastases to the brain. Clin Bull 4(2): 47-57.
- Shaw E, Scott C, Suh J, Kadish S, Stea B, Hackman J, et al. (2003) RSR13 plus cranial radiation therapy in patients with brain metastases: Comparison with the radiation therapy oncology group recursive partitioning analysis brain metastases database. J Clin Oncol 21: 2364-2371.
- Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, et al. (1997) Recursive partitioning analysis (RPA) of prognostic factors in three radiation therapy oncology group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 37(4): 745-51.
- Sirin S, Oysul K, Surenkok S, Sager O, Dincoglan F (2011) Linear accelerator- based stereotactic radiosurgery in recurrent glioblastoma: A single center experience. Vojnosanit Pregl 68(11): 961-966.
- Dincoglan F, Beyzadeoglu M, Sager O, Oysul K, Sirin S (2012) Image-guided positioning in intracranial non-invasive stereotactic radiosurgery for the treatment of brain metastasis. Tumori 98(5): 630-635.
- Dincoglan F, Sager O, Gamsiz H, Demiral S, Uysal B (2012) Management of arteriovenous malformations by stereotactic radiosurgery: A single center experience. UHOD-Uluslararasi Hematoloji-Onkoloji Dergisi 22: 107-112.
- Surenkok S, Sager O, Dincoglan F, Gamsiz H, Demiral S (2012) Stereotactic radiosurgery in pituitary adenomas: A single center experience. UHOD-Uluslararasi Hematoloji-Onkoloji Dergisi 22: 255-260.
- Dincoglan F, Sager O, Gamsiz H, Uysal B, Demiral S, et al. (2012) Stereotactic radiosurgery for intracranial tumors: A single center experience. Gulhane Med J 54(3): 190-198.
- Sager O, Beyzadeoglu M, Dincoglan F, Demiral S, Uysal B, et al. (2013) Management of vestibular schwannomas with linear accelerator-based stereotactic radiosurgery: A single center experience. Tumori 99(5): 617-622.
- Dincoglan F, Beyzadeoglu M, Sager O, Uysal B, Demiral S, et al. (2013) Evaluation of linear accelerator-based stereotactic radiosurgery in the management of meningiomas: A single center experience. J BUON 18(3): 717-722.
- Demiral S, Beyzadeoglu M, Uysal B, Oysul K, Kahya YE, et al. (2013) Evaluation of stereotactic body radiotherapy (SBRT) boost in the management of endometrial cancer. Neoplasma 60(3): 322-327.
- Sager O, Beyzadeoglu M, Dincoglan F, Uysal B, Gamsiz H, et al. (2014) Evaluation of linear accelerator (LINAC)-based stereotactic radiosurgery (SRS) for cerebral cavernous malformations: A 15-year single- center experience. Ann Saudi Med 34(1): 54-58.
- Sager O, Beyzadeoglu M, Dincoglan F, Gamsiz H, Demiral S, et al. (2014) Evaluation of linear accelerator-based stereotactic radiosurgery in the management of glomus jugulare tumors. Tumori 100(2): 184-188.
- Sager O, Dincoglan F, Beyzadeoglu M (2015) Stereotactic radiosurgery of glomus jugulare tumors: Current concepts, recent advances and future perspectives. CNS Oncol 4(2): 105-114.
- Gamsiz H, Beyzadeoglu M, Sager O, Dincoglan F, Demiral S, et al. (2014) Management of pulmonary oligometastases by stereotactic body radiotherapy. Tumori 100(2): 179-183.
- Dincoglan F, Sager O, Gamsiz H, Uysal B, Demiral S, et al. (2014) Management of patients with ≥ 4 brain metastases using stereotactic radiosurgery boost after whole brain irradiation. Tumori 100(3): 302-306.
- Demiral S, Beyzadeoglu M, Sager O, Dincoglan F, Gamsiz H, et al. (2014) Evaluation of linear accelerator (linac)-based stereotactic radiosurgery (srs) for the treatment of craniopharyngiomas. UHOD - Uluslararasi Hematoloji-Onkoloji Dergisi 24: 123-129.
- Gamsiz H, Beyzadeoglu M, Sager O, Demiral S, Dincoglan F, et al. (2015) Evaluation of stereotactic body radiation therapy in the management of adrenal metastases from non-small cell lung cancer. Tumori 101(1): 98-103.
- Dincoglan F, Beyzadeoglu M, Sager O, Demiral S, Gamsiz H, et al. (2015) Management of patients with recurrent glioblastoma using hypofractionated stereotactic radiotherapy. Tumori 101(2): 179-184.
- Demiral S, Dincoglan F, Sager O, Gamsiz H, Uysal B, et al. (2016) Hypofractionated stereotactic radiotherapy (HFSRT) for who grade I anterior clinoid meningiomas (ACM). Jpn J Radiol 34(11): 730-737.
- Dincoglan F, Sager O, Demiral S, Uysal B, Gamsiz H, et al. (2017) Radiosurgery for recurrent glioblastoma: A review article. Neurol Disord Therap 1: 1-5.
- Demiral S, Dincoglan F, Sager O, Uysal B, Gamsiz H, et al. (2018) Contemporary Management of Meningiomas with Radiosurgery. Int J Radiol Imaging Technol 80(2): 187-190.
- Shapiro WR (1999) Current therapy for brain tumors: back to the future. Arch Neurol 56(4): 429-432.
- Sager O, Dincoglan F, Demiral S, Uysal B, Gamsiz H (2018) A concise review of immunotherapy for glioblastoma. Neuroimmunol Neuroinflammation 5: 25.
- Owonikoko TK, Arbiser J, Zelnak A, Shu HK, Shim H (2014) Current approaches to the treatment of metastatic brain tumours. Nat Rev Clin Oncol 11(4):203-22.
- Zakaria R, Das K, Bhojak M, Radon M, Walker C (2014) The role of magnetic resonance imaging in the management of brain metastases: diagnosis to prognosis. Cancer Imaging 14(1): 8.
- Zakaria R, Pomschar A, Jenkinson MD, Tonn JC, Belka C, et al. (2017) Use of diffusion-weighted MRI to modify radiosurgery planning in brain metastases may reduce local recurrence. J Neurooncol 131(3): 549-554.
- Lignelli A, Khandji AG (2011) Review of imaging techniques in the diagnosis and management of brain metastases. Neurosurg Clin N Am 22(1): 15-25.
- Nowosielski M, Radbruch A (2015) The emerging role of advanced neuroimaging techniques for brain metastases. Chin Clin Oncol 4(2): 23.






























