The Potential Prognostic and Diagnostic Role of MiRNAs as Novel Biomarkers for Prostate Cancer
Noha H Ibrahim1, Mona S Abdellateif2*, Yahia M Ismail3, Samar H Kassem4, Marwa Mahmoud Selim3, Asmaa A El-Leithy5 and Mohamed A Abd El Salam6
1Clinical & Chemical Pathology Department, National Cancer Institute (NCI), Cairo University
2Medical Biochemistry & Molecular Biology, Cancer Biology Department, National Cancer Institute (NCI), Cairo University
3Medical Oncology department, National cancer institute (NCI), Cairo University
4Biochemistry division, Laboratory Medicine Department, Faculty of Applied Medical Sciences, 6 October University
5College of Biotechnology, Misr University for Science and Technology (MUST)
6Department of Andrology, Sexology & STDs, Faculty of Medicine, Cairo University
Submission: October 23, 2018; Published: November 16, 2018
*Corresponding Address: Mona S Abdellateif, Medical Biochemistry & Molecular Biology, Cancer Biology Department,National Cancer Institute (NCI),Cairo University, Egypt
How to cite this article: Noha H Ibrahim, Mona S Abdellateif, Yahia M Ismail, Samar H Kassem, Marwa Mahmoud Selim, et al. The Potential Prognostic and Diagnostic Role of MiRNAs as Novel Biomarkers for Prostate Cancer. Canc Therapy & Oncol Int J. 2018; 12(5): 555846. DOI:10.19080/CTOIJ.2018.12.555846
Abstract
Background: The aim of the current study was to assess the potential prognostic role of miRNAs as non-invasive biomarkers in different stages of prostate cancer (PC)./
Methods: Quantitative RT-PCR analysis of plasma miRNAs from 50 PC patients [22 with localized (LPC) and 28 with metastatic disease (MPC)], 20 with benign prostatic hyperplasia (BPH) and 20 healthy normal controls (NC). The markers studied included total and free prostate-specific antigen (PSA), miR-375, miR-378, miRNA-141, and miR-18a./
Results: All markers were highly expressed in MPC and LPC group compared to BPH and NC (P<0.001), with a significant correlation with Gleason score and stage. miR-375 and miR-18a were significantly associated with clinically positive digital rectal examination (DRG), prostate volume, TNM staging of the tumor, lymph node and distant metastasis (P<0.05). The strong association of plasma miR-141, miR-375 and miR-18a with metastatic prostate cancer was observed. The sensitivity of miR-18a, miR-378, miR-375 and FPSA for the diagnosis of LPC was (95.5%, 86.4%, 81.8% and 4.5%; respectively, P<0.001) at 100% specificity. While for diagnosis of MPC, the sensitivity of miR-18a, miR-378, miR-375, miR-141 and FPSA were (60.7%, 53.6%, 78.6%, 53.6% and 96.4%; respectively) at 100% specificity./
Conclusion: circulating plasma miR-18a, miR-375, miR-141 and miR-378 could be used as prognostic markers for the differential diagnosis of localized and metastatic prostate cancer. The clinical utility of these novel biomarkers anticipates extra exploration.
Keywords: miRNAs; Prostate cancer; Biomarkers; Prognosis; PSA
Introduction
Prostate cancer (PC) is considered the second most commonly diagnosed malignancy, and the fifth leading cause of cancer related deaths in males worldwide. It is associated with a variety of risk factors as age, family history, race, hormonal change and genetic abnormalities [1,2].An important prognostic indicator of prostate cancer is the histopathologic grading (Gleason score). Gleason-based grading allows classifying tumors according to their relative degree of differentiation by assigning a score from 1 (most differentiated) to 5 (least differentiated). However, it has been elucidated that tumors with similar histological patterns may provide different clinical outcomes, so Gleason score cannot exactly predict the aggressiveness of the disease although it is a powerful prognostic indicator [3].
prostate-specific antigen (PSA), which is the most widely used biomarker for diagnosis of PC, though it has a low specificity [4].also, using this marker for screening the population leads to over diagnosis of the patients with consequent overtreatment of indolent PC [5,6]. Therefore, identifying new biomarkers thatcould identify and differentiate different stages of PC (localized, advanced and metastatic) is highly needed.
MicroRNAs (miRNAs) are small endogenous single-stranded, non-protein coding RNAs of about 15-22 nucleotides length. They are important regulators of gene expression at the posttranscriptional level because they degrade, or repress, target mRNAs [7]. They have an effect on different cellular processes such as proliferation, apoptosis, differentiation, and regulation of genes expression through modification of messenger RNA (mRNA) [8].
Each individual miRNA may bind up to 200 gene targets, and each gene also may have several binding sites for different miRNAs [9]. Consequently, dysregulation of the expression of miRNA may contribute to the occurrence and progression of cancer. Recent studies demonstrated that miRNAs even if derived from epithelial tumors can be detected in blood, and some circulating miRNAs derived from PC potentially correlate with the risk of disease progression and aggressiveness [10-12]. These studies provided evidence supporting the possible clinical use of circulating miRNAs as non-invasive markers for monitoring disease progression [11,13]. Therefore, the aim of the current study is to assess the potential prognostic role of 4 different circulating miRNAs namely: miR-375, miR-378, miR-141 and miR-18a in patients with PC and their relation to different clinical stages.
Subjects and Methods
This cohort study including 90 subjects; 50 PC patients [22 with localized prostate cancer (LPC) before radical prostatectomy (RP) and 28 with metastatic prostate cancer (MPC)], 20 patients with benign prostatic hyperplasia (BPH) and 20 healthy normal controls (NC). All Patients were histologically and radiologically proven prostate carcinoma who received no medical or surgical therapeutic intervention before enrollment in the study. The exclusion criteria were any severe co-morbidity or prostatitis. The study protocol was approved by the ethical committee of the National Cancer Institute (NCI), Cairo University, Egypt, in accordance with the 2011 declaration of Helsinki. Informed written consent was obtained from each participant before enrolment. PC patients were admitted and treated at urology unit of NCI during the period between June 2015 and January 2018. BPH patients were recruited from Andrology unit at Kasr Al-Ainy hospital, and the NC subjects were healthy volunteers.
All subjects underwent routine laboratory investigations and imaging diagnosis. Staging of PC patients was done by using the TNM staging system [14]. Tumor aggressiveness was detected by histological tumor grading system in the Gleason score with ≤ 7 considered low grade, and score >7 was considered highgrade. Peripheral blood samples (7ml) were withdrawn from PC patients before the start of any active treatment and divided into two tubes: the first containing k2EDTA for plasma separation and the second for serum collection. Fresh blood samples on k2EDTA were immediately centrifuged at room temperature for 10 min at 3000 R.P.M. The plasma samples were then liquated and frozen at ˚C 80 until RNA extraction. Serum collecting tubes were left to clot for 30 minutes and then centrifuged at 4000 R.P.M for 10 minutes. Serum was then used for the determination of total prostatespecific antigen (TPSA) and free prostate-specific antigen (F.PSA) by chemiluminescence assays (Architect i1000SR Immunoassay Analyzer, Abbott, and the U.S.A) according to the manufacturer’s instructions.
RNA Extraction and Reverse Transcription (RT)
Total RNA including miRNA was separated from 200μL of plasma using the miRNeasy Mini Kit [cat. no. 217004, Qiagen, Germany] according to the manufacturer’s recommended protocol.Concentration and purity of RNA samples were measured using Nanodrop 1000 [Thermo Scientific NanoDropTM spectrophotometer ND 1000 Wilmington USA]. Then, The RNA was eluted in 40μL of RNase- free water and stored at -80°C until reverse transcription (RT) reactions.
Reverse transcription of the total RNA (100 ng) was carried out using mi Script II RT Kit [catalog no. 218161 Qiagen, Germany] according to the manufacturer’s instructions.complementary DNA (cDNA) was then synthesized in a thermal cycler [IGEM: MIT/2005/Thermo cycler]. The cDNA was stored at -80°C until. Quantitative Real-Time Polymerase Chain Reaction2.5μl of cDNA was amplified using 10μl of TaqMan 2X Universal PCR Master Mix II [Applied Biosystems; Thermo Fisher Scientific], 1μl of gene-specific primers of the target miRNA and 6.5μl of nucleasefree water in a final volume of 20μl. qPCR was run on the Step One Real-Time PCR system [Applied Biosystems, Foster City, CA, USA].The reaction mixtures were incubated at 95˚C for 10 min to stimulate HotStarTaq DNA polymerase, followed by 40 cycles: (denaturation for 15 s at 94 °C, annealing for 30 s at 55°C, finally extension for 30 s at 70°C).
The expressions of selected miRNAs in the blood were normalized to the expression of U6 small nuclear RNA (RNU6B). The data obtained from the miRNA expression levels were evaluated by the cycle threshold (Ct) method [15].ΔCt was calculated by subtracting the Ct values of RNU6B from the Ct values of the target miR-RNA. ΔΔCt was then calculated by subtracting the average ΔCt of the healthy control samples from the ΔCt of the patient’s samples (LPC, MPC, and BPH). The fold change in the miRNA expression level was calculated (fold change = 2-ΔΔCt) to determine the relative quantitative levels of target miRNA [16].
Statistical Analysis
All data were statistically analyzed using SPSS software version 25 [IBM SPSS, Armonk, NY, USA]. Data were presented as median and range. Comparisons between patients’ groups were analyzed using Kruskal- Wallis test, and Mann- Whitney. MPC, and BPH. Receiver operating characteristic (ROC) curves were used to assess miRNAs as biomarkers, and the area under the curve (AUC) was reported. P value < 0.05 (two-tailed) was considered statistically significant.
Results
This is a retrospective cohort study conducted on 22 patients with localized prostate cancer (LPC), twenty-eight patients with metastatic prostate cancer (MPC) and 20 cases with benign prostate hyperplasia (BPH), compared to 20 normal control group (NC).
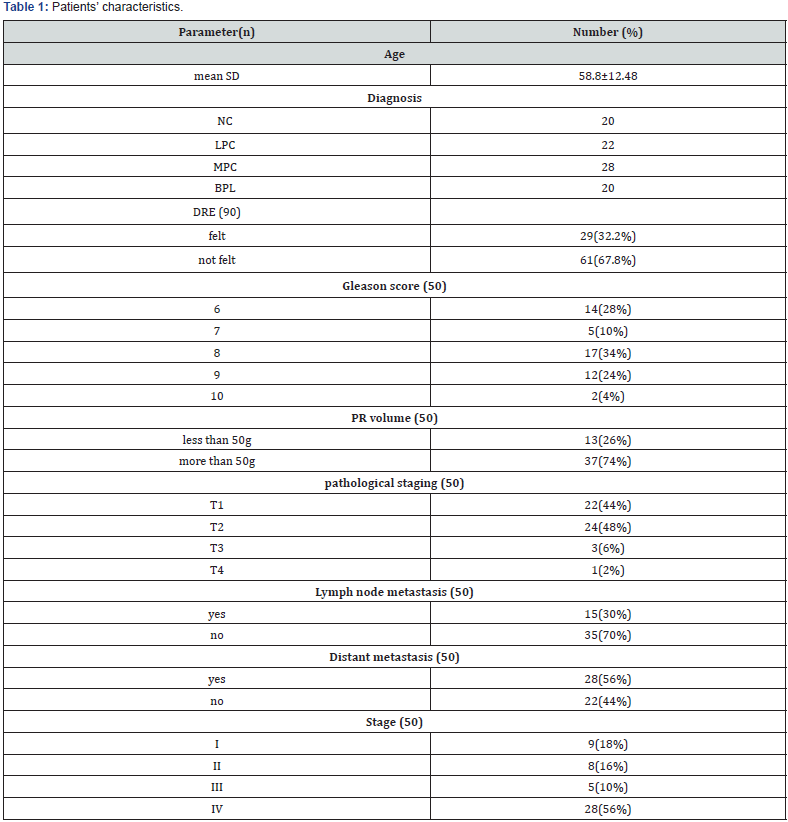
Patients’ characteristics
The mean age of the patients was 58.8±12.48. Of all 50 cancer patients, 9 (18%) cases were stage I, eight (16%) were stage II, five (10%) cases were stage III, and 28 (56%) patients were stage IV. Positive digital rectal examination (DRE) was felt in 29 (32.2%) cases. Gleason score was 6 in 14 (28%) of patients, 8 in 17 patients (34%), and 9 in 12 (24%) patients, while only 2 (4%) patients had Gleason score 10. Prostate volume was more than 50g in 37 (74%) patients. 15 (30%) patients had lymph node metastasis while 28 (56%) patients had distant metastasis (Table 1).
Plasma expression of miRNAs and PSA
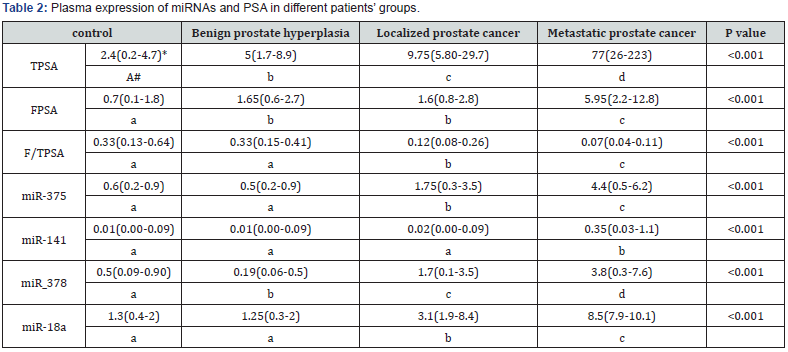
There was a statistically significant difference among all patients’ groups regarding TPSA plasma expression (P<0.001). However, regarding FPSA; there was no statistically significant difference between BPH groups and LPC (P=0.91), while there was a significant difference among NC, LPC and MPC groups (P<0.001). Also, regarding F/TPSA; there was no statistically significant difference between NC and BPH groups (P=0.56), while there was a significant difference among NC, LPC and MPC groups (P<0.001).
There was no statistically significant difference between NC and BPH groups regarding plasma expression of miR-141, miR- 375 and miR-18a (P = 0.083, 0.661 and 0.684; respectively). Moreover, there were significant differences among LPC, MPC and non-malignant groups (NC and BPH) regarding plasma expression of miR-141, miR-375 and miR-18a (P<0.001, for all), however there was no significant difference in the expression level of miR- 141 between LPC and non-malignant groups (NC and BPH). There was a statistically significant difference among all tested groups; NC, BPH, LPC, and MPC ((P<0.001) regarding miR-378 expression (Table 2)(Figure 1).
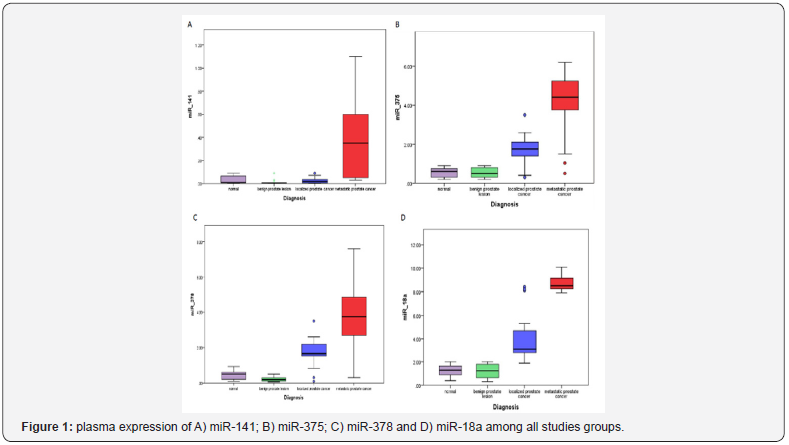
ROC curve analysis for the tested miRNAs and FPSA in localized prostate cancer and non-malignant cases.
As relative median miRNAs expression was differentially expressed in the peripheral blood of PC patients, BPH patients and control subjects. The potential of peripheral blood oncogenic ROC curve analysis was performed for FPSA, miR-375, miR-378, and miR-18a to differentiate patients with LPC and those with BPH and NC. MiR-375 showed AUC, 0.919; 95% CI, 0.828-1.000 (P<0.0001), miR-378 showed AUC, 0.939; 95% CI, 0.857-1.000 (P<0.0001), and FPSA showed the lowest AUC, 0.704; 95% CI, 0.577-0.831 (P<0.008). MiR-18a showed the highest AUC, 0.996; 95% CI, 0.987-1.000 (P<0.0001). The sensitivity of miR-18a, miR- 378, miR-375 and FPSA were (95.5%, 86.4%, 81.8% and 4.5%; respectively) at 100% specificity (Figure 2A).
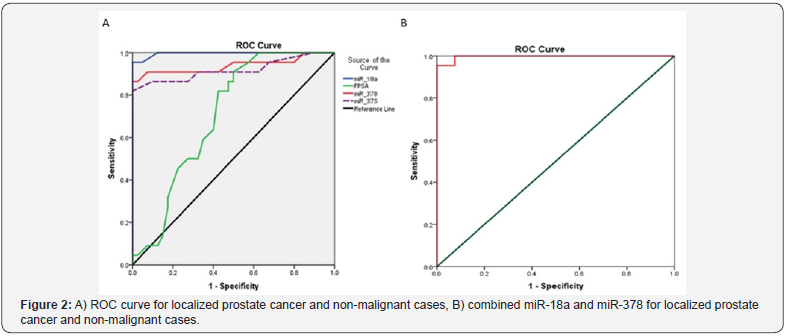
By adding miR-378 to miR-18a for detecting patients with LPC, the sensitivity (95.5%) didn’t increase at a specificity 100 %, AUC was 0.997 and 95%CI was 0.988-1.0; P<0.0001 (Table 3) (Figure 2B).ROC curve analysis for the tested miRNAs in localized prostate cancer and metastatic cases.ROC curve analysis was performed for FPSA, miR-375, miR-141, miR-378 and miR-18a to differentiate patients with LPC and those with MPC. It showed that FPSA had the highest AUC, 0.996; 95% CI, 0.986-1.000 (P<0.0001). miR-375 showed AUC, 0.911; 95% CI, 0.817-1.000 (P<0.0001). MiR-141 showed AUC, 0.925; 95% CI, 0.854-0.996 (P<0.0001). MiR-18a showed AUC, 0.966; 95% CI, 0.922-1.000 (P<0.0001). While miR-378 showed the lowest AUC, 0.828; 95% CI, 0.704-0.952 (P=0.002). The sensitivity of miR-18a, miR-378, miR-375, miR-141 and FPSA were (60.7%, 53.6%, 78.6%, 53.6% and 96.4%; respectively) at 100% specificity (Figure 3A).


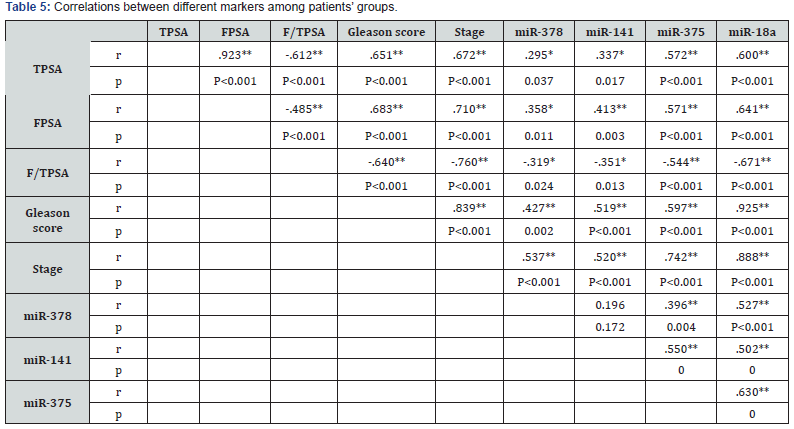

By combining FPSA and miR-378 for differentiating patients with MPC, it didn’t affect the sensitivity (96.4%) at a specificity 100%, AUC is 0.982 and 95%CI is 0.946-1.0; P<0.0001 (Table 4) (Figure 3B).Correlations between miRNAs, PSA and clinicpathological features of the patients.There was significant correlation between the tested miRNA (miR-18a, miR-378, miR- 375 and miR-141) with PSA, FPSA, Gleason score and pathological stages of the tumor (P<0.01). while there was significant inverse correlation with F/TPSA (P<0.05, (Table 5).
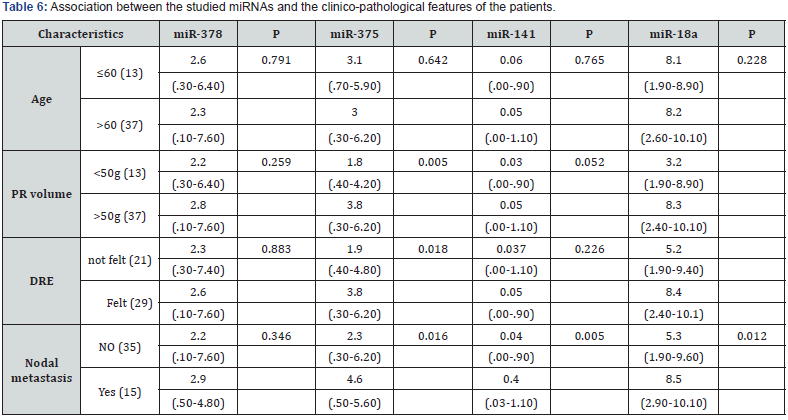
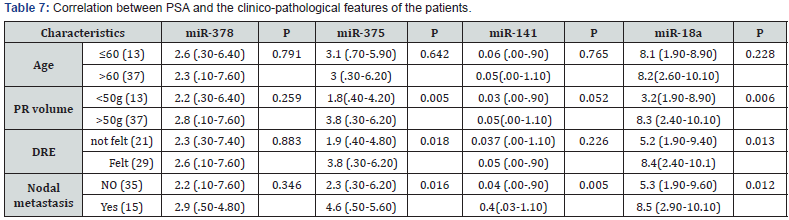
miR-375, miR-18a, TPSA, FPSA, and F/TPSA were significantly associated with PR volume (P=0.005, 0.006, 0.001, 0.001 and 0.025; respectively), DRE (P=0.018, 0.013, 0.003, 0.006 and 0.007; respectively) and nodal metastasis (P=0.016, 0.012, 0.001, 0.001 and 0.002; respectively). Also, miR-141 associated significantly with nodal metastasis (P=0.005). miR-378 and miR-141 didn’t associate significantly with age, PR volume and DRE (P>0.05) (Tables 6&7).
Discussion
miRNAs play an important promising role in the prediction and progression of prostate cancer [17]. In the current study, we investigated the role of miR-375, miR-378, miR-141 and miR- 18a in the diagnosis of LPC and MPC compared to BPH and NC subjects.Our results demonstrated that the expression levels miR-375, miR-378 and miR-18a follow an increasing trend with disease progression. These data agreed with Nguyen et al. [18] who demonstrated an elevated expression of circulating miR- 375, miR-378 and miR-141 in patients with metastatic castration resistant prostate cancer compared to those with low-risk LPC. However, our data regarding the expression level of miR-141 is controversial with other several studies, we found that miR-141 is only significantly expressed in MPC, while there was no significant difference between LPC and benign groups (BPH and NC). Our data were consistent with that reported by Agaoglu et al. [19]who reported that plasma miR-141 could only distinguishes localized from metastatic prostate cancer patients [19], as miR-141 is a member of the miR-200 family, which has an essential function in epithelial-to-mesenchymal transition [20,21].
However, it was in contrary to Mitchell et al. who demonstrated elevated expression of circulating miR-375 and miR-141 in patients with prostate cancer in comparison to healthy control [11]. Similarly, other previous studies reported increased expression of miR-375 and miR-141 in prostate cancer patients with advanced disease compared to patients with earlier stages of prostate cancer or healthy tissues [8,10,22, 23]. miRNA-375 potentially down-regulates Sec23A in prostate cancer cell lines resulting in enhanced proliferation, indicating that miR-375 may have a role in promoting cell growth [24].
Regarding miRNA18a, we found increased expression of miRNA18a in LPC and MPC patients compared to normal control. These data were consistent with that reported by He et al. [25]. Hence, MiR-18a target a serine/threonine-protein kinase 4 that acts as a tumor suppressor in prostate cancer [26]. The upregulation of miR-18a binds directly to the 30UTR of the STK4 mRNA, and by downregulating STK4 at the protein level, it thus suppresses apoptosis and promotes tumor survival [27]. We also found a significant association of miR-375 and miR- 18a with clinically positive digital rectal examination (DRG), prostate volume, TNM staging of the tumor, distant and lymph node metastasis which confirm their possible uses as prognostic markers for PC patients.Our data showed increased serum FPSA level in metastatic groups and thus provide an easy and simple way for detection of patients with metastasis. Therefore, it can be used in the follow up of the disease progression, not only in early detection of PC in patients with grey zone PSA level as approved by FDA (5-9).
This was confirmed by assessing the diagnostic power of the tested miRNAs together with FPSA. we found that FPSA could differentiate accurately patients with MPC with 96.4% sensitivity and 100% specificity, followed by miR-375 which achieved 78.6% sensitivity and 100% specificity[28]. Whereas for the diagnosis of patients with LPC, our results showed that miR-18a could detect LPC patients with 95% sensitivity and 100% specificity. As PC is a heterogeneous and complex disease, thus it is potentially needed to search for a panel of markers, including miRNAs that could help in diagnosing of such patients.
Conclusion
In conclusion, our results confirmed the great potential of circulating miR-18a, miR-375, miR-141 and miR-378 as prognostic biomarkers for the differential diagnosis of metastatic prostate cancer from localized indolent prostate cancer, which may produce clinical different aspects of prostate cancer management, and thus deserve further investigation in a large scale study to further confirm its usefulness in the identification of occult micrometastasis which is too small to be detected with conventional imaging techniques. additional studies of these miRNAs’ roles in pathogenesis of dissemination of metastases will still be needed to developed targets to future curative therapy of PC.
References
- Crawford ED (2003) Epidemiology of prostate cancer. Urology 62: 3-12.
- Witte JS (2009) Prostate cancer genomics: towards a new understanding. Nat Rev Genet 10: 77-82.
- Dhok A (2017) Role of Serum PSA in Diagnosis of Prostate Cancer with Histopathological Correlation and Predictive Value of Serum PSA in Early Detection of Prostate Cancer. Journal of Medical science and clinical research 5(7).
- Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, et al. (2003) The influence of finasteride on the development of prostate cancer. N Engl J Med 349: 215-224.
- Ciatto S, Zappa M, Bonardi R, Gervasi G (2000) Prostate cancer screening: the problem of overdiagnosis and lessons to be learned from breast cancer screening. Eur J Cancer 36(11): 1347-1350.
- Croce CM (2009) Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet 10(10): 704 -714.
- Dall’Era MA, Cooperberg MR, Chan JM, Davies BJ, Albertsen PC, et al. (2008) Active surveillance for early-stage prostate cancer: a review of the current literature. Cancer 112: 1650-1659.
- Lewis BP, Burge CB, Bartel DP (2005) Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120(1):15-20.
- Bartel DP (2004) MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 116: 281-297.
- Krek A, Grun D, Poy MN, Wolf R, Rosenberg L, et al. (2005) Combinatorial microRNA target predictions. Nat Genet 37(5):495-500.
- Brase JC, Johannes M, Schlomm T, Falth M, Haese A, et al. (2011). Circulating miRNAs are correlated with tumor progression in prostate cancer. Int J Cancer 128(3):608-616.
- Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, et al. (2008) Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U SA 105(30): 10513-10518.
- Selth LA, Townley S, Gillis JL, Ochnik AM, Murti K, et al. (2011) Discovery of circulating microRNAs associated with human prostate cancer using a mouse model of the disease. Int J Cancer 131(3): 652-661.
- Chen, X, Ba, Y, Ma, L, Cai X, Yin, Y, et al. (2008) Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell research 18(10): 997-1006.
- Sobin LH, Wittekind CH (2002) TNM Classification of Malignant Tumours, (6th), Wiley Liss, New York, USA p: 184-187.
- Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3(6): 1101-1108.
- Livak K, J.and Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2- ΔΔCT method. Methods, 25 (4): 402-408.
- Luu, H N, Lin, H Y, Sørensen, KD, Ogunwobi, OO, Kumar N, et al. (2017) miRNAs associated with prostate cancer risk and progression. BMC urology 17(1): 18.
- Nguyen, HC N, Xie W, Yang, M, Hsieh C, et al. (2013) Expression differences of circulating microRNAs in metastatic castration resistant prostate cancer and low‐risk, localized prostate cancer. The Prostate 73(4): 346-354.
- Agaoglu, FY, Kovancilar M, Dizdar Y, Darendeliler E, Holdenrieder S, Dalay, N, Gezer U (2011). Investigation of miR-21, miR-141, and miR-221 in blood circulation of patients with prostate cancer. Tumor Biology 32(3): 583-588.
- Griffiths-Jones, Saini HK, Van Dongen S, Enright AJ, Mirbase (2008) Tools for microrna genomics. Nucleic Acids Res 36: D154-158.
- Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, et al. (2008) The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nature cell biology 10(5): 593-601.
- Wach S, Nolte E, Szczyrba J, Stöhr R, Hartmann A, et al. (2012) MicroRNA profiles of prostate carcinoma detected by multiplatform microRNA screening. Int J Cancer 130(3): 611-621.
- Szczyrba J, Löprich E, Wach S, Jung V, Unteregger G, et al. (2010) The microRNA profile of prostate carcinoma obtained by deep sequencing. Mol Cancer Res 8(4): 1541-7786.
- Szczyrba J, Nolte E, Wach S, Kremmer E, Stöhr R, et al. (2011) Downregulation of Sec23A protein by miRNA-375 in prostate carcinoma. Mol Cancer Res 9(6): 791-800.
- He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, et al. (2005) A microRNA polycistron as a potential human oncogene. nature 435(7043): 828-833.
- Hsu TI, Hsu CH, Lee KH, Lin JT, Chen CS, et al. (2014) MicroRNA-18a is elevated in prostate cancer and promotes tumorigenesis through suppressing STK4 in vitro and in vivo. Oncogenesis 3(4): e99.
- Minoo P, Zlobec, I, Baker K, Tornillo L, Terracciano L, et al. (2007) Prognostic significance of mammalian sterile20-like kinase 1 in colorectal cancer. Modern pathology 20(3): 331-338.






























