Preliminary Results of Clinical Outcomes with Hypofractionated Intensity Modulated Radiotherapy in Organ Confined Prostate Cancer: An Indian Experience
Jyotirup Goswami1, Suman Mallik1, Monidipa Mondal1*, Saikat Sheet1, Sayan Das1, Arijit Sen1, Bipasha Pal2, Suresh Das3 and Soura Palit3
1Radiation Oncology Department, Narayana Superspeciality Hospital, India
2Radiation Physics&Radiation OncologyDepartment, Narayana Superspeciality Hospital, India
3Medical Physicist& Radiation Oncology Department, Narayana Superspeciality Hospital, India
Submission: August 11, 2018; Published: August 31, 2018
*Corresponding Address: Monidipa Mondal, Junior Consultant, Radiation Oncology Department, Narayana Superspeciality Hospital, 120/1 Andul road, Howrah, West Bengal-711103, India, Tel: +91 9836396483; Email: monidipamondal1@gmail.com
How to cite this article: Jyotirup Goswami, Suman Mallik, Monidipa Mondal, Saikat Sheet, et al. Preliminary Results of Clinical Outcomes with Hypofractionated Intensity Modulated Radiotherapy in Organ Confined Prostate Cancer: An Indian Experience. Canc Therapy & Oncol Int J. 2018; 11(5): 555823. DOI: 10.19080/CTOIJ.2018.11.555823
Abstract
Introduction: Recent evidences suggest that hypofractionated radiotherapy (HFRT) has comparable clinical outcome as conventionally fractionated radiotherapy in organ-confined prostate cancer. We hereby report our initial data of clinical outcomes of organ-confined prostate cancer patients treated with HFRT
Material and Methods: 45 consecutive organ-confined prostate cancer patients (1 low- risk, 5 intermediate- risk, and 39 high-risk) received hypofractionated intensity modulated radiotherapy (HF-IMRT), from July 2012 to June 2017. The prescribed dose to the prostate was 77Gy/35 fractions (before 2014, n=8) or 60Gy/20 fractions (from 2014 onwards, n=37). Irradiation of pelvic lymph nodes was done where required. Androgen deprivation therapy was given for 6 months in intermediate risk and 24-36 months in high risk patients. Biochemical relapse free survival (bRFS) [Phoenix definition], prostate cancer-specific and overall survival (pCSS and OS) actuarial curves were assessed using Kaplan Meier survival curve. Acute and late toxicities were recorded according to the RTOG morbidity scoring system.
Results: Median follow was 26 months (range, 6-54 months). 6 biochemical relapses occurred (1 in intermediate and 5 in high risk group) of whom 3 died of distant metastasis. The 2-year actuarial bRFS was 92.3%, pCSS was 93.9% and OS was 91.5%. RTOG grade 2 or worse acute and late gastrointestinal toxicities were 20% and 13.3%; genitourinary toxicity were 8.9% and 11.1% respectively. Only 1 patient had Grade 3 rectal toxicity, and none had Grade 3 bladder toxicity.
Conclusion: HF-IMRT in our settings has a comparable biochemical relapse rate and toxicity profile with that of the published literature.
Keywords: Dose Hypofractionation; Intensity-Modulated Radiotherapy; Prostate Neoplasms; Treatment Outcome
Abbreviations: HFRT: HypofractionatedRadiotherapy; HF-IMRT: HypofractionatedIntensity Modulated Radiotherapy; bRFS- Biochemical Relapse Free Survival; pCSS: Prostate cancer-specific survival; OS: Overall Survival; RTOG: Radiation Therapy Oncology Group; CFRT: Conventionally Fractionated Radiotherapy; NCCN: National Comprehensive Cancer Network; PSA: Prostate Specific Antigen; EORTC: European Organization for Research and Treatment of Cancer; CTV:Clinical Target Volume; PTV: Planning Target Volume; VMAT: Volume Modulated Arc Therapy; QUANTEC: Quantitative Analysis Of Normal Tissue Effects In The Clinic; EQD2- Equivalent Dose in 2 Gy per Fraction; SPSS: Statistical Package for Social Sciences; SD: Standard Deviation;BED:Biological Effective Dos; GI/GU: Gastrointestinal/Genitourinary; ADT: Androgen Deprivation Therapy; TURP: Trans Urethral Resection of Prostate
Introduction
Radiotherapy is a mainstay of treatment for organ-confined prostate cancer. In low-risk disease, surgery in the form of radical prostatectomy is equivalent, whereas in intermediate- & high-risk disease, radiotherapy is combined with short-course (6-month) & long-course (2-3 years) androgen deprivation therapy, respectively
Dose escalation to prostate, up to 81.6 Gy, resulted in higher disease control but was also associated with increased late rectal toxicity[1,2].Studies have estimated theα/β ratio of prostate to be 1.5Gy which is significantly less than the α/β ratio of 3 Gy for late complications in rectal tissue, the dose limiting organ in prostate radiotherapy[3,4].Therefore HFRT schedules are expected to provide better late toxicity profile with logistic advantage of fewer number of fraction and should, if appropriately dosed, also lead to equivalent oncologic outcomes for appropriate target dosage. Use of conformal radiotherapy techniques, particularly image guided, and intensity modulated radiotherapy, has permitted the use of hypofractionated radiotherapy with tolerable late bladder and bowel toxicity
Results from recently published randomized studies [5- 8] have shown that hypofractionated intensity modulated radiotherapy (HFRT) has comparable clinical outcome as conventionally fractionated radiotherapy (CFRT) in organ confined prostate cancer and therefore it can become a new standard of care. Though different schedules of HFRT are lately being practiced in India, very few Indian centers have reported their clinical outcome with HFRT in Carcinoma Prostate. We therefore report our preliminary results of clinical outcome with hypofractionated intensity modulated radiotherapy in Organ confined Carcinoma Prostate.
Materials and Methods
Study Design
The study included 45 consecutive patients of organ-confined prostate cancer who received radical radiotherapy at our centre between July 2012-June2017.This included patients of all three risk categories, defined as per the National Comprehensive Cancer Network (NCCN) guidelines[9],thus: low risk= T1- T2a with Gleason score =< 6 and PSA< 10 ng/ml; intermediate risk=T2b-2c with Gleason score 7 and PSA 10-20 ng/ml and high and very high risk=T3-4 or Gleason score>=8 or PSA >20 ng/ ml.The patients of intermediate-risk disease received 6 months of androgen deprivation therapy with LHRH analogues and were taken up for immediate radiotherapy. The patients of highrisk disease were to receive 2-3 years of androgen deprivation therapy and were taken up for radiotherapy after 3 months of commencement of the same.
Simulation and Treatment Procedure
All patients underwent CT-based planning. Wherever possible, a MRI scan of the pelvis was ordered as well for the purpose of image-fusion for delineation. For the planning CT scan, intravenous contrast was used whenever the patient’s renal function was within normal limits. The patient was asked to void his bladder and drink 500cc of water, following which he was taken to the scanner and positioned supine, using a kneerest (VacLoc was used in some cases). The LASER fiducials were marked on the skin and 3mm slices were taken on the Somatom Emotion [Siemens AG, Germany] 16 slice scanner, from the level of L3 vertebra to the mid-thigh.
The images were then transferred to the contouring workstation (Focal Sim version 4.80.02 and MONACO Sim version 5.11) [Elekta AB, Sweden], where delineation of targets and normal structures were done. Where available, the transverse T2-W MRI images were fused with the planning CT images. The normal structures delineated were the rectum, sigmoid colon, small bowel (as a space), bilateral femoral heads and penile bulb. The Clinical Target Volume 1 (CTV1) included the prostate without margins for low-risk disease, while for intermediate-risk, the retro-pubic space was included as well as the base/whole of the seminal vesicles for high-risk disease when indicated. The CTV 2 included the prophylactically irradiated bilateral pelvic nodes in case of high-risk disease or pelvic node positive disease, as per our institutional protocol. The Planning Target Volume 1 (PTV1) included the CTV1 with a 7 mm craniocaudal and 7mm radial margin except 5 mm towards rectum(in those with 3D image guidance), while the PTV2 included the CTV2 with a 7mm isotropic margin.
Treatment planning was done on the CMS XiO/MONACO 3D Treatment Planning Systems [Elekta AB, Sweden]. The prescribed dose to the prostate was 77Gy/35 fractions (before 2014, n=8) or 60Gy/20 fractions (from 2014 onwards, n=37). In case of high-risk disease or node positive disease, pelvic lymph node received a dose of 50Gy/25 fractions or 44Gy/20 fractions respectively. Both forward & inverse planned IMRT (step and shoot) and Volume Modulated Arc Therapy (VMAT) were used, using 6 MV X-ray beam. The plan acceptance criteria for target coverage were that 95% of the PTV would be covered by 95% of the prescribed dose and no volume of the PTV would receive a dose above 107% of the prescribed dose.For organs-at-risk, the QUANTEC criteria were used: salient among these were that 15%, 25%, 35% and 50% of the rectum would receive less than 75Gy, 65Gy, 60Gy, 50Gy respectively, while corresponding doses for urinary bladder were 80Gy,75Gy, 70Gy, 65Gy, while the volume of small bowel receiving 45Gy or more would be less than 192cc (all doses in EQD2)[10].
Each plan thus accepted was subjected to plan-specific Quality Assurance using the iMatrixx phantom (an ion chamber array), Octavius 4D phantom and Qasar phantom, keeping the gamma value = 3% for acceptance.The patients underwent treatment on the 6MV linear accelerator, using the same bladder-filling protocol mentioned above, as for the simulation procedure. For image-verification with either electron portal imaging or kilovoltage cone beam CT was done daily for the first 5 days; online correction was done & appropriate action taken for systemic errors; subsequently, pre-treatment imaging was repeated daily and online corrections were implemented to reduce random variations. During treatment, patients were reviewed weekly and as required, using the RTOG/ EORTC acute radiation morbidity scoring scheme[11] to record toxicities. Following completion of treatment, patients underwent a PSA assessment and clinical examination every 3 months. The RTOG/EORTC late radiation morbidity scoring scheme11 was used to record late toxicities (beyond 3 months of completion of treatment).
Definition of Clinical Outcomes
Biochemical relapse free survival (bRFS), defined as the time interval from the first day of radiotherapy to biochemical relapse according to the Phoenix definition (PSA concentration greater than nadir plus 2 ng/mL)[12]; prostate cancer specific survival (pCSS), defined as the time interval from the first day of radiotherapy to prostate cancer related death i.e death from a clinical/biochemical progression; overall survival (OS), defined as the time interval from the first day of radiotherapy to death from any cause or censoring at date of the last follow up.
Statistical Analysis
Statistical analysis was done using Statistical Package for Social Sciences (SPSS) version 20.0 (IBM Inc, USA). Kaplan- Meier curves were used to generate various time to event curves and log-rank statistical test was applied to determine the difference between actuarial rates. Univariate analyses were performed to estimate the risks of bRFS, pCSS and OS using the known prognostic factors: Gleason score, pretreatment PSA level, T stage, risk group, Radiotherapy fractionation schedule. Maximum grade of acute and late RTOG toxicity was reported in percentage.
Result
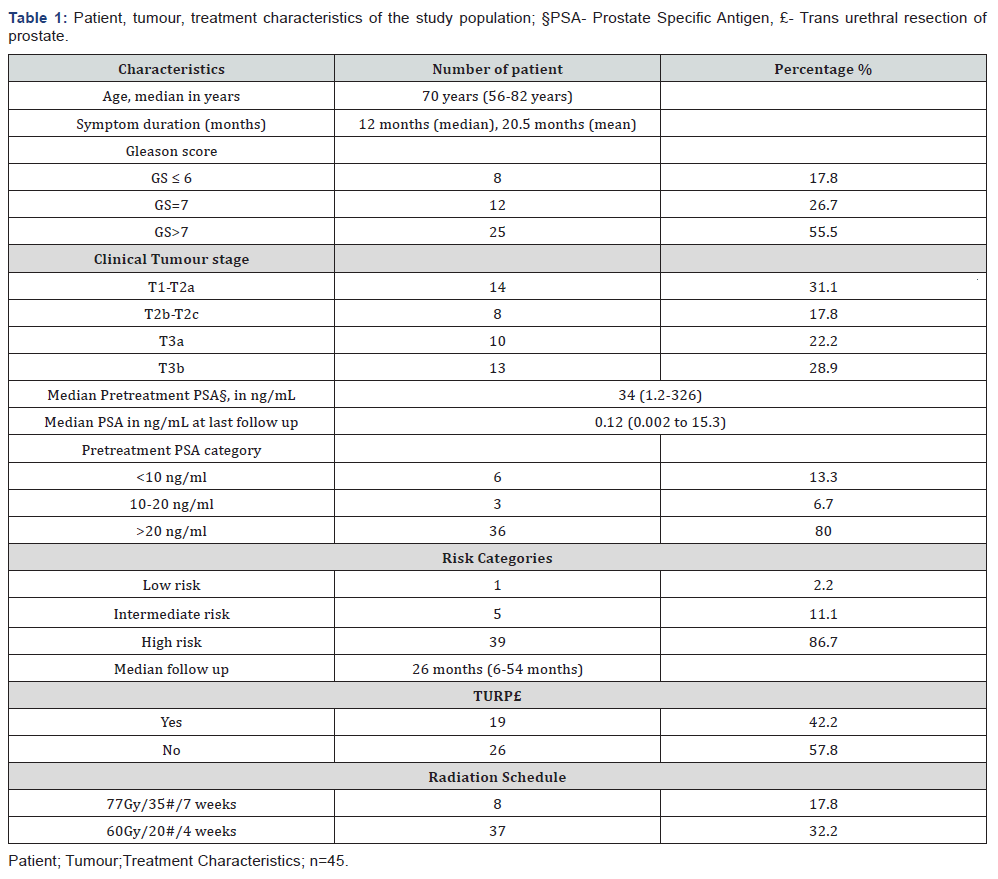
Patient and Tumor and Treatment Characteristics
Clinical outcomes of 45 consecutive patients of organconfined prostate cancer were analyzed in this study. Out of 45 patients, 1 patient was of low- risk [LR], 5 patients of intermediate- risk [IR], and 39 patients of high-risk [HR]. Patient, tumor and treatment characteristics are summarized in Table 1. Median follow was 26 months (range, 6-54 months).Median age was 70 years (56-82 years) and median PSA value was 34 ng/ ml (range, 1.29 to 326.5ng/ml) at presentation and 0.12 ng/ml (range, 0.002 to 15.3ng/ml)at last follow up.Eight patients were treated with 77Gy/35 fractions/7 weeks schedule (before 2014) and 37 patients were treated with 60Gy/20 fractions/4 weeks schedule (after 2014). 39 patients received nodal irradiation (6 patients received 50Gy/25 fractions and 33 patients received 44Gy/20 fractions to the pelvic nodes. All patients tolerated radiotherapy well and completed radiotherapy as per schedule. The median duration of ADT was 22 months (among the patients who have finished ADT).
Dosimetric Outcome
Mean PTV volume (in percentage) receiving 95% of the prescribed dose (V95%) was 96.15%. (SD-1.7). Mean dose received by 95% of the PTV (D95%) was 97.6% of the prescribed dose. (SD- 3.7).Mean bladder dose was 48.1Gy and that of rectum was 45.3Gy. Dose to 1% volume of the bladder and rectum was 67.8Gy and 64.8Gy respectively.V75, V70, V65 of bladder were 6.3%, 11.2% and 15.2% respectively and V75, V70, V65, V60 of rectum were 4.2%, 14.8%, 23.6% and 28.7 % respectively. Other dosimetric parameters are show in Table 2.

Clinical outcome statistics
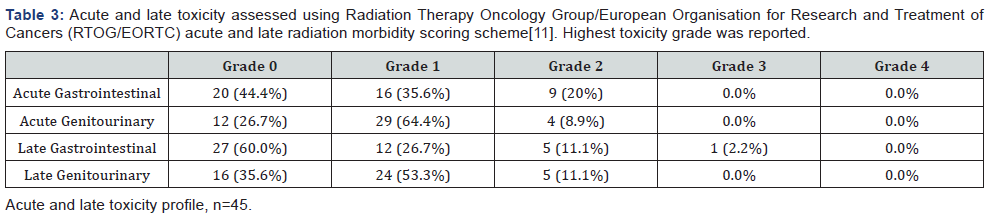
After a median follow was 26 months (range, 4-54 months), 6 biochemical relapses occurred (1 in intermediate risk group and 5 in high risk group) of which 3 died of distant metastasis. The overall 2-year actuarial bRFS was 92.3%, pCSS was 93.9% and OS was 91.5% and 3-year actuarial bRFS, pCSS and OS rates were 78%, 90%, 87.7% respectively. The survival curve for bRFS is shown in Figure 1. On Univariate analysis, higher T stage (≥2b) and pretreatment PSA level showed a trend towards difference in bRFS, though did not attain statistical significance (p=0 .18, p=0.17, respectively).The acute RTOG toxicity profile is shown in Table 3. Acute Grade 0,1 and 2 GU toxicities were 26.7%, 64.4% and 8.9% respectively and GI toxicities were 44.4%, 35.6% and 20% respectively. Grade 3 or 4 acute GI/GU toxicity was not reported. At median follow up of 26 months, the rate of physician assessed RTOG grade 2 or worse late gastrointestinal and genitourinary toxicity were 13.3% and 11.1% respectively. Only 1 patient had Grade 3 rectal toxicity and none had Grade 3 bladder toxicity.

Discussion
The radiobiological basis of hypofractionation in carcinoma prostate comes from the study conducted by Brenner and Hall to extract the sensitivity of prostatic tumors to changes in fractionation[3].Result from the study showed the estimated α/β value being 1.5 Gy which is lower than the dose limiting surrounding normal tissue. They concluded that hypofractionation schemes would be expected to have same tumour control rate and late toxicity but reduced acute toxicity with the logistic and financial advantages of fewer numbers of fractions[3].Since then large number of studies have been conducted comparing clinical outcomes of different hypofractionated radiotherapy schedules with that of conventionally fractionated schedules. This data of hypofractionated radiotherapy has been further strengthened by the recently published four randomized studies.
The CHHiP trial is the largest (n=3216), three-arm, noninferiority trial which compared two modest hypofractionated radiotherapy schedules (60Gy/20 fractions/4 weeks); and 57Gy/19 fractions/3•8 weeks) with a conventionally fractionated schedule (74Gy /37 fractions/7.4 weeks). After a median follow-up of 62•4 months, 5-year biochemical or clinical failure-free rates were 90•6% in 60Gy/20 fractions schedule; 85•9% in 57Gy/19 fractions schedule and 88•3% with conventional fractionation schedule ;the 60 Gy hypofractionation schedule was shown to be non-inferior to the 74 Gy conventional fractionation regimen of 74Gy (HR 0•84, 90% CI 0•68–1•03; pNI=0•0018)[13].Though acute toxicity peaked sooner with hypofractionation compared with conventional fractionation with, there were no differences in long-term toxicity or patientreported outcomes[14].
Another randomized trial (PROFIT trial) compared the 60Gy in 20 fractions hyofractionated regimen with 78Gy/39 fractions conventional regimen in intermediate risk prostate cancer without androgen deprivation therapy. The biochemical failure event rate at 5 years was 21% in both the arms and acute genitourinary/gastrointestinal toxicity more than grade 3 was comparable in both the arm; however late toxicity favored the hypofractionated arm (3.5% vs. 5.4%)[15]. Results from these two randomized trials provide compelling evidence that the 60 Gy in 20 fractions hypofractionated regimen is as effective and safe as that of the conventional fractionated regimens.
The NRG Oncology 0415 trial,[5] also tested the noninferiority of hypofractionated IFRT over conventional fractionation but in low-risk prostate cancer and with different hypofractionation regimen. They compared 73.8 Gy/41 fractions (CFRT) with 70 Gy/28 fractions (HFRT). After a median followup was 5.8 years the estimated 5-year disease-free survival was 85% for CFRT and 86% for HFRT; cumulative incidence of biochemical recurrence at 5 years was 8% and 6% respectively with increased late (more than grade2) gastrointestinal/ genitourinary (GI/GU) adverse events with hypofractionation (HFRT 22%/30%: CFRT 14%/23%)[5].The authors concluded that this HFRT schedule was non-inferior to CFRT, although with an increased risk of late toxicity. It should be noted here that the biological effective dose (BED3) of the hypofractionated regimen in NRG Oncology 0415 was 128•33Gy which is slightly greater than that of the conventional regimen (118.08Gy) which could have accounted for late GI/GU toxicity.
HYPRO trial,[6] the largest superiority trial (n=804) tested the hypothesis that HFRT of 64.9Gy (19 fractions of 3•4 Gy, three fractions per week) would increase 5-year relapse-free survival by 10%, from 70% to 80%, compared with conventional fractionation (CFRT)of 78 Gy (39 fractions of 2•0 Gy, five fractions per week) in intermediate and high-risk prostate cancer. The 5-year bRFS rates were 81% and 77% (HR 0.86; P=0.36) respectively and superiority was not proven. The acute toxicity results of this trial showed that 3 months after radiotherapy, Grade 2 or worse GI/GU toxicity was 13%/23% in HFRT arm and 13%/22% in CFRT arm[16].The cumulative incidence of grade 3 or more late genitourinary toxicity was higher with HFRT (19% vs 13% with CFRT), but the incidence of grade 2 or more bowel toxicity at 3 years was similar[17].
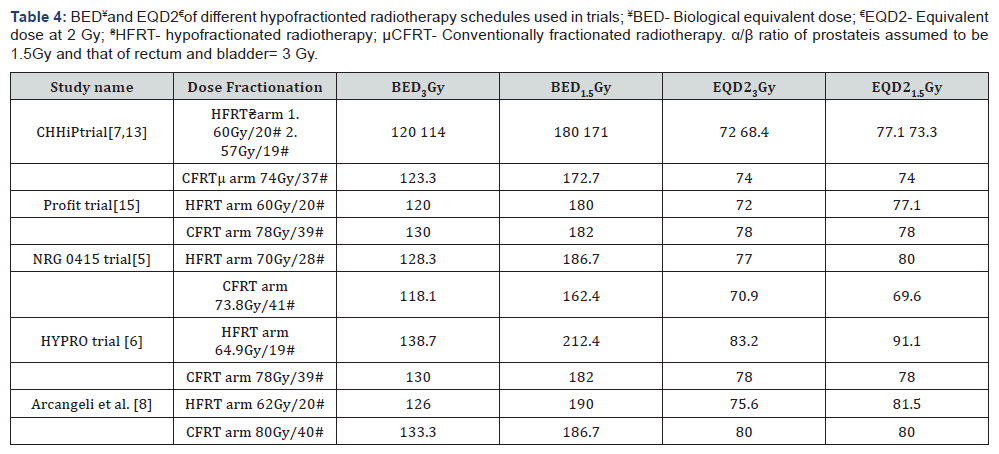
Arcangeli et al. [8] randomised 168 cases of high risk prostate cancer to HFRT (62 Gy in 20 fractions in 5 weeks) and CFRT (80 Gy in 40 fractions in 8 weeks). The 10-year FFBF (freedom from biochemical failure) rate was 72% in the HFRT and 65% in the CFRT arm (P = .148). At a median follow-up of 9 years there was no difference between physician-assessed late Grade 2 or worse GI/GU toxicity. The biological equivalent dose (BED) and equivalent dose at 2 Gy (EQD2) of different fractionation schedules used in these randomized studies with α/β value 1.5 and 3 are shown in Table 4. Results from CHHiP and PROFIT trial showed that 60Gy/20fractions HFRT regimen was as effective as CFRT and was also safe in terms of acute and late toxicity. The trials using hyopfractionated radiotherapy schedules with higher BED (NRG 0415 and HYPRO) showed higher percentage of late GI/GU toxicity and failed to show non-inferiority of HFRT. In our institution, from 2014 onwards we have been using the HFRT regimen of 60Gy/20 fractions after the publication of preliminary results of CHHiP trial, before which 77Gy/35 fractions scheduled was followed.
Although HFRT is now starting to be widely practiced in India there are very few published data of clinical outcomes of HFRT in India. The first Indian study by Murthy et al. [18] reported clinical outcome with helical tomotherapy-based HFRT for prostate cancer. They used different hypofractionated regimen with the BED3 ranging from 110-129 Gy with 32% of the patient (17out of 53) receiving 60Gy/20fraction schedule. 83% patients (44 out of 53) were high risk and median duration of ADT received was 9 months (among those who have finished). At a median follow up of 23 months, biochemical relapse-free survival rate was 95.2%, 3-year actuarial bRFS rate was 78.4% and OS was 95%. In our study, the patient population was almost similar (i.e 86% patients were of high risk) and the same 3-year actuarial bRFS rate was achieved, although the median duration of ADT received was higher in our study (22 months). Randomised trials[19,20] have shown that long term androgen deprivation results in improved biochemical control and overall survival in high risk prostate cancer patients and therefore higher 3-year bRFS rate was expected from our study compared to the previous study, which did not occur in practice. This could be probably explained by the fact that 39% of the patient in previous series received higher BED1.5 (i.e BED1.5>180Gy) which could have resulted in similar bRFS. The RTOG Grade 2 or worse acute GI toxicity rate of both the study was also comparable (20% in present study versus 26.4% in previous study), but Grade 2 or worse GU toxicity was lesser with our study.
A recently published phase 1/2 study from India on stereotactic hypofractionated once-weekly radiation therapy in patients with on metastatic prostate cancer has shown encouraging results. 30 patients were treated with a dose of 35 Gy in 5 fractions delivered once-weekly by volumetric intensity modulated arc therapy. Incidence of Grade 2 or worse acute GI/ GU toxicities was low (3.3%, and 0%respectively)[22]. With a median follow-up of 25 months, 2-year biochemical control rate was 96.7%. This approach will need further analysis before it can be accepted as standard practice.
To summarise, modestly hypofractionated radiotherapy has generally shown equivalent (but not superior) oncologic outcomes with comparable acute and late toxicities as conventionally fractionated radiotherapy. Our study results confirm these hypotheses and bear out earlier published Indian data on the same; given the paucity of Indian data, we believe this study will be an important addition to the published literature. The limitations of the present study include its retrospective nature, relatively small sample size and limited length of followup. Patient reported toxicity outcomes were not analysed in this study.
Conclusion
Hypofractionated radiotherapy using IMRT in the Indian scenario has a comparable biochemical relapse profile with that of the published literature. The acute bladder and rectal toxicity profile are also acceptable. Modest hypofractionated radiotherapy should become the new standard of care, though longer follow-up will benefit our understanding of the long-term toxicity profile[6].
Acknowledgement
None.
Conflicts of Interest
None.
References
- Beckendorf V, Guerif S, Le Prisé E, Cosset JM, Bougnoux A, et al. (2011) 70 Gy versus 80 Gy in localized prostate cancer: 5-year results of GETUG 06 randomized trial. Int J Radiat Oncol Biol Phys 80(4): 1056- 1063.
- Chism DB, Horwitz EM, Hanlon AL, Pinover WH, Mitra RK, et al. (2003) Late morbidity profiles in prostate cancer patients treated to 79–84 Gy by a simple four-field coplanar beam arrangement. Int J Radiat Oncol Biol Phys 55(1): 71-77.
- Brenner DJ, Hall EJ (1999) Fractionation and protraction for radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys 43(5): 1095-1101.
- Fowler JF (2005) The radiobiology of prostate cancer including new aspects of fractionated radiotherapy. Acta Oncologica 44(3): 265-276.
- Lee WR, Dignam JJ, Amin MB, Bruner DW, Low D, et al. (2016) Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low-risk prostate cancer. Journal of Clinical Oncology 34(20): 2325-2332.
- Incrocci L, Wortel RC, Alemayehu WG, Aluwini S, Schimmel E, et al. (2016) Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO): final efficacy results from a randomised, multicentre, open-label, phase 3 trial. The Lancet Oncology 17(8): 1061-1069.
- Dearnaley D, Syndikus I, Sumo G, Bidmead M, Bloomfield D, et al. (2012) Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: preliminary safety results from the CHHiP randomised controlled trial. The lancet oncology 13(1): 43-54.
- Arcangeli G, Saracino B, Arcangeli S, Gomellini S, Petrongari MG, et al. (2017) Moderate hypofractionation in high-risk, organ-confined prostate cancer: final results of a Phase III randomized trial. Journal of Clinical Oncology 35(17): 1891-1897.
- NCCN Clinical Practice Guidelines in Oncology (2017) Prostate Cancer, version 2.2017-February 21, 2017.
- Bentzen SM, Constine LS, Deasy JO, Eisbruch A, Jackson A, et al. (2010) Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC): an introduction to the scientific issues. Int J Radiat Oncol Biol Phys 76(3): S3-S9.
- Cox JD, Stetz J, Pajak TF (1995) Toxicity criteria of the radiation therapy oncology group (RTOG) and the European organization for research and treatment of cancer (EORTC). Int J Radiat Oncol Biol Phys 31(5): 1341-1346.
- Roach M, Hanks G, Thames H, Schellhammer P, Shipley WU, et al. (2006) Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 65(4): 965-974.
- Dearnaley D, Syndikus I, Mossop, H Khoo V, Birtle A, et al. (2016) Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol 17(8): 1047-1060.
- Wilkins A, Mossop H, Syndikus I, Khoo V, Bloomfield D, et al. (2015) Hypofractionated radiotherapy versus conventionally fractionated radiotherapy for patients with intermediate-risk localised prostate cancer: 2-year patient-reported outcomes of the randomised, noninferiority, phase 3 CHHiP trial. The Lancet Oncology 16(16): 1605- 1616.
- Catton CN, Lukka H, Julian JA, Gu CS, Martin J, et al. (2016) A randomized trial of a shorter radiation fractionation schedule for the treatment of localized prostate cancer. The Lancet Oncology 34: A5003.
- Aluwini S, Pos F, Schimmel E, Van Lin E, Krol S, et al. (2015) Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): acute toxicity results from a randomised non-inferiority phase 3 trial. The Lancet Oncology 16(3): 274-283.
- Aluwini S, Pos F, Schimmel E, Krol S, Van der Toorn PP et al. (2016) Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): late toxicity results from a randomised, non-inferiority, phase 3 trial. The Lancet Oncology 17(4): 464-474.
- Murthy V, Krishnatry R, Mallik S, Master Z, Mahantshetty U, et al. (2013) Helical tomotherapy-based hypofractionated radiotherapy for prostate cancer: a report on the procedure, dosimetry and preliminary clinical outcome. Journal of cancer research and therapeutics 9(2): 253-260.
- Zapatero A, Guerrero A, Maldonado X, Alvarez A, San Segundo CG, et al. (2015) High-dose radiotherapy with short-term or long-term androgen deprivation in localised prostate cancer (DART01/05 GICOR): a randomised, controlled, phase 3 trial. The Lancet Oncology 16(3): 320-327.
- Horwitz EM, Bae K, Hanks GE, Porter A, Grignon DJ, et al. (2008) Tenyear follow-up of radiation therapy oncology group protocol 92-02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol 26(15): 2497-2504.
- Arunsingh M, Mallick I, Prasath S, Arun B, Sarkar S, et al. (2017) Acute toxicity and its dosimetric correlates for high-risk prostate cancer treated with moderately hypofractionated radiotherapy. Medical Dosimetry 42(1): 18-23.
- Mallick I, Arunsingh M, Prasath S, Arun B, Nallathambi C, et al. (2017) Phase 1/2 Study on Stereotactic Hypofractionated Once-Weekly Radiation Therapy for Nonmetastatic Prostate Cancer. Int J Radiat Oncol Biol Phys 99: S156.






























