Clinical Case of Malignant Acrospiroma, Treatment Results Evaluation
*Hojouj Mohammad IM, I Bondarenko, V Zavizion VL Maltseva and Ievgen Domanskiy
Department of Oncology and Medical Radiology, Dnipropetrovsk medical academy of Health Ministry of Ukraine, Europe
Submission: August 13, 2017; Published: August 22, 2017
*Corresponding author: Hojouj Mohammad IM, Department of Oncology and Medical Radiology at Dnipropetrovsk Medical Academy Dnipropetrovsk Multi-filed Clinical Hospital #4 SE”, Municipal Institution "Dnipropetrovsk City Multi-field Clinical Hospital #4”, Volodymyr Vernadskii str., 9, Dnipro, 49044, Ukraine, Europe, Email: Hojouj@yahoo.com
How to cite this article: Hojouj M I, I Bondarenko, V Zavizion V M, Ievgen D. Clinical Case of Malignant Acrospiroma, Treatment Results Evaluation. Canc Therapy & Oncol Int J. 2017; 8(2): 555694. DOI: 10.19080/CTOIJ.2017.06.555694
Introduction
Malignant acrospiroma (MA) - is rather rare tumor with eccrine ductal and secretory differentiation, including clear cell component. This type of tumor appears more often in elderly people (average male patient age is 51 years, female patient - 55 years). The tumor corresponds to solitary intradermal, exophytic or mixed type node 0.5-2 cm or more in diameter, hemispheric, tight-elastic texture, on wide base, covered with unchanged skin, sometimes with ulcers. Small percent of cases have clear discharge from tumor. Considering the same architecture of malignant nodular hydradenoma and benign analogue, it is difficult to find the difference between them, although undisputed manifestation of malignance is vessel and perineural invasion, lymphogenic metastasis. The tumor is very aggressive and liable to metastasis. It is important to notice that there are no direct histological and clinical signs, predicting biological behavior of the tumor. Despite chemotherapy, patients with metastasis has fatal outcome vary rapidly. There no proved data of chemotherapy impact on malignant acrospiroma (Figure 1).
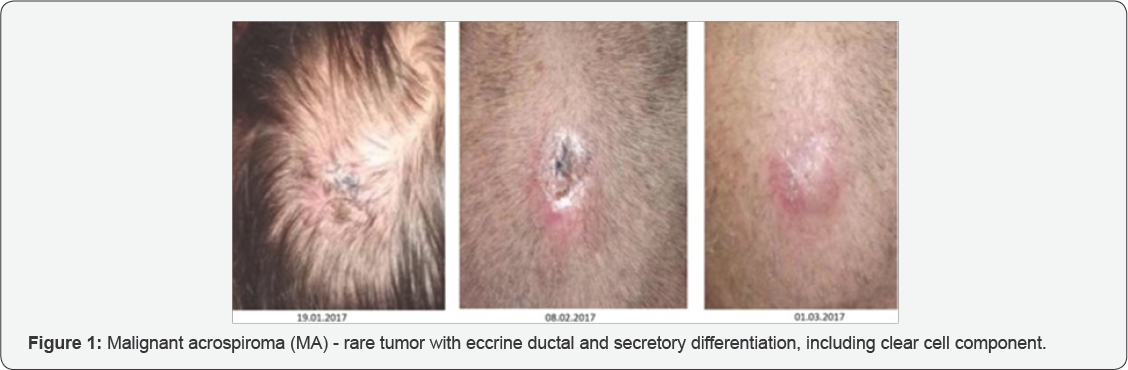
Description of MA Clinical Case
Patient, 58 years, applied to Dnipropetrovsk Multi-field Clinical hospital #4 in Jan 2017 to the Department of Oncology and Medical Radiology of Dnipropetrovsk Medical Academy with complaints to fatigue and tumor formation.
Anamnesis
Patient considers he is sick since autumn 2016, when above mentions complaint appeared for the first time. Incision biopsy was performed in Nov 2016, and then the patient was sent to the Department of Oncology and Medical Radiology of the Dnipropetrovsk Multif-field Clinical Hospital #4. Pathohistological conclusion as of 16 Nov 2016: Tumor has the structure of malignant eccrine acrospiroma with ulceration.
Life History
Catarrhal diseases are 1-2 times per year. No addictions. No allergies. No hemotrasfusions, traumas and surgeries. Diabetes mellitus type II, more than 2 years. No cancer family history.
Physical Examination
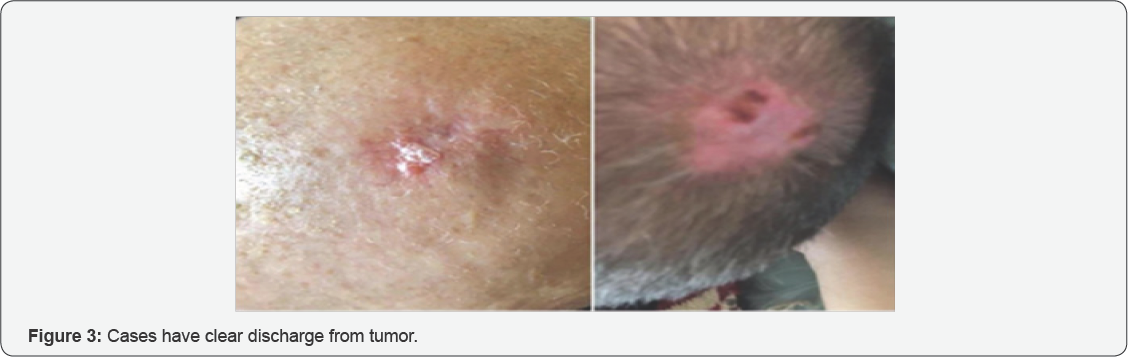
Formation in left parietal region, round in shape with indeterminate boundaries, 2 cm in diameter, with tissue lysis and perifocal inflammation (Figures 2 & 3).

Investigation Data
i. Pathohistological conclusion N 10291-96/16 as of 16 Nov 2016: Tumor has the structure of malignant eccrine acrospiroma with local ulceration.
ii. X-ray assessment of the chest as of 11 Jan 2017: Radiological signs of metastasis in lungs.
iii. Endocrinologist assessment as of 13 Jan 2017: Diabetes mellitus type II, compensated.
iv. Therapeutist assessment as of 13 Jan 2017: Essential hypertension II gr. Coronary heart disease: atherosclerotic cardio sclerosis, Heart failure I.
v. CT assessment of chest, abdominal cavity and pelvis as of 17 Jan 2017: CT-signs of multiple secondary changes on lungs, increased mediastinal lymph nodes, metastasis in liver S4. Cholelithiasis. Concernments in right kidney. Mass lesion in left lobe of thyroid gland.
vi. CT assessment of brain as of 17 Jan 2017: CT-signs of encephalopathy.
vii. Echocardiography as of 20 Jan 2017: Satisfactory myocardial contractility, LVEF - 60%
viii. Punctate from thyroid gland as of 26 Jan 2017: Accumulation of polymorphic cells of follicular epithelial tissue, stroma elements.
ix. CA results as of 20 Jan 2017: 19.05 U/mL.
x. 10 Hematology as of 13 Jan 2017 - HB 120 g/L - RBC 4.08 x 10^12/L - WBC 7.56 x 10^9/L - SOE 28 mm/h - lymphocytes 28.7 % - monocytes 11.8%.
xi. Hematology was done 2 days before chemotherapy and 5 days after. Patient had chemotherapy-induced leucopenia and neutropenia grade I-II CTC AE, recovered after drug administration. No chemotherapy cycles delayed.
xii. Hematology as of 03 Jul 2017 - HB 134 g/L - RBC 4.58 x 10^12/L - WBC 5.34 x 10^9/L - SOE 8 mm/h - lymphocytes 28.7 % - monocytes 6%.
xiii. Chemistry as of 13 Jan 2017 - total bilirubin - 7.3 mmol/L - ALT - 21 IU/L - AST - 23 IU/L - total protein - 55.8 g/L - alkaline phosphatase - 74 IU/L.
xiv. RW as of 13 Jan 2017 - negative.
xv. AID as of 13 Jan 2017 - negative.
xvi. Hepatitis B and C as of 13 Jan 2017 - negative.
Diagnosis
Malignant acrospiroma, T2N0M1 stage IV (mts in lungs and liver), clinical group 2 Clinical case was discussed on board of doctors. Chemotherapy was prescribed. Received treatment:
1. Carboplatin - VISTA AUG 6
2. Docetaxel - VISTA 75 mg/m2 IV 3. Fluorouracil - VISTA 500 mg/m2 IV
3. Visual clinical dynamics in the course of the treatment.
Recommendation at Discharge
i. Family doctor supervision.
ii. Control blood analysis in 7-21 days.
iii. CT assessment of chest, abdominal cavity and pelvis after 3 months.
Complete Diagnosis at Discharge
Malignant acrospiroma, T2N0M1 stage IV (mts in lungs and liver), condition after non-radical surgery, after 8 cycles of chemotherapy clinical group II. Patient had positive dynamics after chemotherapy treatment, complete response as per RECIST 1.1. Patient had not complaints. CT assessment of chest, abdominal cavity and pelvis with IV contrast as of 28 Jun 2017. CT-signs of stable size of thyroid gland left lobe formation, positive dynamics due to disappearance of lesions in lungs, mediastinal lymph nodes, and liver lesions. No new lesions. Patient has fully active lifestyle, and he is under family doctor and oncologist supervisio






























