Anchorage-Independent Tumor Cells Clustering and Implication in Metastatic Dissemination
Fabien Gava1, Bernard Ducommun1,2* and Valérie Lobjois1*
1Université de Toulouse, ITAV, CNRS, UPS, Toulouse, France
2CHU de Toulouse; F-31059 Toulouse, France
Submission: July 24, 2017; Published: July 27, 2017
*Corresponding author: Valérie Lobjois and Bernard Ducommun, Centre Pierre Potier, ITAV-USR3505, 1 place Pierre Potier, F-31106 Toulouse Cedex 1, France, Tel: +33582991010; Email: valerie.lobjois@itav.fr; bernard.ducommun@itav.fr
How to cite this article: Gava F, Ducommun B, Lobjois V. Anchorage-Independent Tumor Cells Clustering and Implication in Metastatic Dissemination. Canc Therapy & Oncol Int J. 2017; 6(2): 555683. DOI: 10.19080/CTOIJ.2017.06.555683
Abstract
How tumor cell clustering is regulated and contributes to cancer dissemination remains unclear. The detection of clusters of circulating tumor cells (CTCs) over expressing cell-cell adhesion proteins have been correlated with high metastatic potential and poor prognosis. This observation strengthens the need for further understanding on the tumor cell aggregation process and how it could contribute to cancer progression. Our laboratory developed an original live microscopy-based methodology and identified regulators of the early step of cancer cells aggregation including E-cadherin and desmosomal proteins. Here we briefly review our current knowledge on the involvement of cellcell adhesion proteins in anchorage-independent cell clustering. We discuss how these findings enlighten the research on the mechanism of metastatic dissemination.
Keywords: Tumor cells clustering; Aggregation; Cell-cell adhesion proteins; Metastasis
Abbreviations: EMT: Epithelial-to-mesenchymal transition; CTC: Circulating Tumor Cell; DSG2: Desmoglein 2; DSC2: Desmocolin 2
Introduction
Metastasis formation implies evasion of cancer cells from the primary site, local invasion, intravasation into blood and lymphatic vessels, extravasation at distant sites and formation of metastatic deposits that will develop as metastatic lesions [1]. Cancer cells dissemination and invasion capabilities have been prominently associated with the activation in carcinoma cells of the epithelial-to-mesenchymal transition (EMT) program. The EMT program induces morphological and motility changes of cells and repression of the expression of cell-cell adhesion molecules [1]. However, an alternative model for cancer cells dispersion has also recently been proposed. It involves escape from the primary tumor of cells that have retained epithelial differentiation markers and that collectively disseminate or subsequently aggregate to form clusters, as suggested by the detection of clusters of circulating tumor cells in blood samples from metastatic patients [2-4]. Aggregation and formation of clusters are among the strategies developed by tumor cells that have evaded from a primary tumor to prevent anoikis in absence of anchorage [5] and the formation of multicellular clusters has been shown to prolong survival of cancer cells [6]. Clusters of CTCs have 23- to 50-fold increased metastatic potential than isolated CTCs and are associated with adverse outcomes. Circulating tumor micro emboli (CTM) associating cancer cells with platelets, stromal cells, and hematopoietic cells, might also protect tumor cells from apoptosis [7]. Thus, CTCs clusters greatly contribute to the metastatic spread of cancer. However, so far, only a few regulators of tumor cell aggregation have been identified and how this process contributes to tumor growth and metastatic dissemination still remains unclear.
It has been demonstrated several years ago that increased expression of E-cadherin, a major component of the cell adherens junctions, enhances cell aggregates formation [8], a result consistent with E-cadherin considered as an invasion suppressor [9] and with its loss of function associated with metastasis [1,9]. However, increased adhesion properties of cancer cells have also been associated with a higher experimental metastatic potential in vivo [10,11] and unexpected roles for E-cadherin in favoring tumor dissemination has been proposed [1,9]. Several links have been made between E-cadherin expression, tumor cell aggregation and cell proliferation. Loss of adhesion normally leads to cell cycle arrest in G1 [12], unless the cells have became anchorage-independent and it was shown that the cyclin-dependent kinase inhibitor p21WAF1 promotes anchorage- independent growth of HCT116 colon carcinoma cells via E-cadherin expression to form large clusters [13]. Similarly in prostate cancer cells, E-cadherin-dependent aggregation has been reported to be associated with Rb-mediated G1 arrest and survival [14].
Circulating clusters of tumor cells have been detected in patients' blood samples and the work by Aceto et al. [2] enlighten a key relation between high metastatic potential and elevated expression of the plakoglobin desmosomal adhesion protein in circulating tumor cells (CTCs). In patients with breast cancer, the cell junction component plakoglobin was identified as highly differentially expressed, and in mouse models, knockdown of plakoglobin abrogates CTCs cluster formation and suppresses lung metastases [2]. Hence, a model of metastatic dissemination that emphasizes the role of tumor cell clusters that have retained or gained epithelial properties with high expression of cytoskeletal and adhesion protein such as E- and P-cadherins was recently proposed [4].
Tumor cells aggregation regulation involves cell-cell adhesion proteins, including members of the cadherin super family [8] as well as other cell surface associated proteins such as MUC1 or Galectin-3 for instance [6]. With the aim of characterizing quantitatively the aggregation capability of a tumor cell population and to identify additional regulators, our laboratory developed a highly reproducible time-lapse microscopy-based quantification assay to monitor the kinetics of aggregation of tumor cells in the absence of cell - substrate adhesion [15]. As cells aggregate and form a cluster, the area decreases in a highly reproducible manner. Automatic detection and tracking of the forming aggregate was performed using custom dedicated software (Figure 1). We used this area variation parameter to quantify the anchorage-independent cell aggregation kinetic in response to pharmacological agents or after siRNA transfection to identify mechanisms involved in cell aggregation. Using this approach, we showed that in HCT-116 colon cancer cells not only are E-cadherins involved in aggregation, but also desmoglein (DSG2) and desmocolin (DSC2), two desmosomal proteins, work in parallel. We also showed that decreased cytoskeleton tension correlated with faster aggregation, suggesting that myosin IIa applies a counter force that slows down aggregation [15]. Accordingly, a few studies have also suggested that cell aggregation correlates inversely with tension in the cytoskeleton [6,16].
Our cancer cells aggregation assay was also validated with breast tumor cell lines including the MCF-7 cell line and is currently being used to investigate the involvement of several other actors and regulatory pathways in this process using dedicated micro devices and time-lapse video microscopy approaches. We present in Figure 1 the effect of the inhibition or invalidation of E-Cadherin using antibodies and siRNA interference strategies. These data emphasize the central role played by E-Cadherin in the aggregation process in MCF7 breast cancer cells. Very recently a chemotaxis-driven model for aggregation of tumor cells has also been proposed [17], suggesting that paracrine cell-cell communications could also been involved in these process (Figure 1).
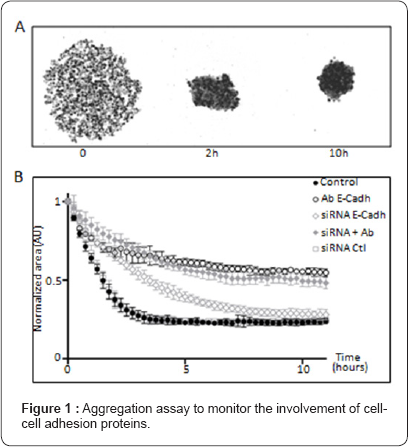
As already published [15], when seeded in condition preventing cell adhesion to the substrate, i.e. in anchorage-free condition, breast adenocarcinoma MCF7 cells progressively clusterize to form a solid shaped aggregate within 10 hours (panel A). This assay allows precise monitoring and quantification of the involvement of E-Cadherin in the aggregation process (Panel B) using monoclonal antibody (Ab E-Cadh), siRNA (siRNA E-Cadh) and the combination of both (siRNA + Ab). Control untreated and scrambled control siRNA (siRNA ctl) are also shown. Normalized cluster area is expressed in arbitrary unites (AU).
Conclusion
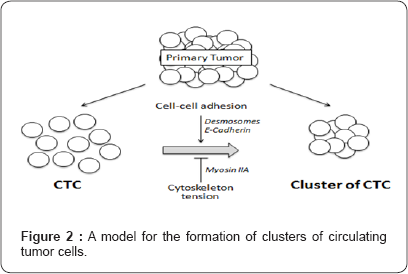
How tumor cell clustering contributes to cancer development and how it is regulated remain unclear. Loss of E-cadherin, a master cell-cell adhesion protein, is a hallmark of epithelio-mesenchymal transition associated with metastasis. However, the detection of clusters of circulating tumor cells (CTCs) over expressing cell-cell adhesion proteins in patients has been correlated to high metastatic potential and poor prognosis. These results illustrate how unexpectedly [18] cellcell interaction key players are involved in collective migration and CTC formation (Figure 2). Clusters of circulating tumor cells (CTC) can derive from direct migration in the blood stream of a group of associated cells that have escaped the primary tumor, or can originate from gathering of single CTCs. Cell adherens junctions and desmosomal proteins act as inducer while cytoskeleton tension inhibits aggregation in the absence of anchorage. Adapted from [15].
These observations of pro- or anti-tumor role for cell aggregation strengthen the need for further investigation on the regulatory mechanisms and how the balance between increasing and decreasing cell aggregation capabilities could contribute to cancer progression. Ongoing studies dedicated to identifying original cell mechanisms that control cancer cells clustering will undoubtedly foster research on the mechanism of metastatic dissemination and might open new therapeutic opportunities.
Acknowledgement
We are grateful to the members of our group for their interest to this project. The support of the staff of the ITAV imaging facility is gratefully acknowledged. The authors wish to acknowledge the TRI-Genotoul facilities. The work in our laboratory is financially supported by the Région Midi-Pyrénées, the Cancer Plan and la Ligue Nationale Contre le Cancer (Comité de la Haute-Garonne).
References
- Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5): 646-674.
- Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS et al. (2014) Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 158(5): 1110-1122.
- Cheung KJ, Padmanaban V, Silvestri V, Schipper K, Cohen JD, et al. (2016) Polyclonal breast cancer metastases arise from collective dissemination of keratin 14-expressing tumor cell clusters. Proc Natl Acad Sci USA 113(7): E854-863.
- Cheung KJ, Ewald AsJ (2016) A collective route to metastasis: Seeding by tumor cell clusters. Science 352: 167-169.
- Guadamillas MC, Cerezo A, Del Pozo MA (2011) Overcoming anoikis- -pathways to anchorage-independent growth in cancer. J Cell Sci 124: 3189-3197.
- Zhao Q, Barclay M, Hilkens J, Guo X, Barrow H, et al. (2010) Interaction between circulating galectin-3 and cancer-associated MUC1 enhances tumour cell homotypic aggregation and prevents anoikis. Mol Cancer 9: 154.
- Dive C, Brady G (2017) SnapShot: Circulating Tumor Cells. Cell 168: 742-742.
- Foty RA, Steinberg MS (1997) Measurement of tumor cell cohesion and suppression of invasion by E- or P-cadherin. Cancer Res 57(22): 5033-5036.
- Shamir ER, Ewald AJ (2015) Adhesion in mammary development: novel roles for E-cadherin in individual and collective cell migration. Curr Top Dev Biol 112: 353-382.
- Topal B, Roskams T, Fevery J, Penninckx F (2003) Aggregated colon cancer cells have a higher metastatic efficiency in the liver compared with nonaggregated cells: an experimental study. The Journal of surgical research 112(1): 31-37.
- Updyke TV, Nicolson GL (1986) Malignant melanoma cell lines selected in vitro for increased homotypic adhesion properties have increased experimental metastatic potential. Clin Exp Metastasis 4(4): 273-284.
- Kang JS, Krauss RS (1996) Ras induces anchorage-independent growth by subverting multiple adhesion-regulated cell cycle events. Mol Cell Biol 16(7): 3370-3380.
- Mueller S, Cadenas E, Schonthal AH (2000) p21WAF1 regulates anchorage-independent growth of HCT116 colon carcinoma cells via E-cadherin expression. Cancer Res 60(1): 156-163.
- Day ML, Zhao X, Vallorosi CJ, Mathew Putzi, C Thomas Powell, et al. (1999) E-cadherin mediates aggregation-dependent survival of prostate and mammary epithelial cells through the retinoblastoma cell cycle control pathway. J Biol Chem 274: 9656-9664.
- Saias L, Gomes A, Cazales M, Ducommun B, Lobjois V (2015) Cell-Cell adhesion and cytoskeleton tension are key but opposing regulators of tumor cell aggregation. Cancer Research 75(12): 1-8.
- Derycke L, Stove C, Vercoutter-Edouart AS, De Wever O, Dolle L, et al. (2011) The role of non-muscle myosin IIA in aggregation and invasion of human MCF-7 breast cancer cells. Int J Dev Biol 55: 835-840.
- Puliafito A, De Simone A, Seano G, Gagliardi PA, Di Blasio L, et al. (2015) Three-dimensional chemo taxis-driven aggregation of tumor cells. Sci Rep 5: 15205.
- Cheung KJ, Ewald AJ (2014) Illuminating breast cancer invasion: diverse roles for cell-cell interactions. Curr Opin Cell Biol 30: 99-111.






























