The Sonographic Assessment of Kidneys in Patients with Sickle Cell Disease
Mohamed A Eltahir1,2*, Moawia Gameraddin1, Elsafi A Abdallah2, Mahmoud S Babiker1, Mohamed Elhaj3, Suliman Salih1,4, and Mohamed E M Gar-elnabi1
1Department of Diagnostic Radiological Technology, Taibah University, Saudi Arabia
2Faculty of Radiological Sciences, Sudan University, Sudan
3Faculty of Alghad, Almadinah, Kingdom of Saudi Arabia
4National Cancer Institute, University of Gezira, Sudan
Submission: January 09, 2017; Published: February 03, 2017
*Corresponding author: Moawia Bushra Gameraddin, Department of Diagnostic Radiological technology, faculty of Applied Medical Sciences, Taibah University, Almadinah, Kingdom of Saudi Arabia, Tel:00966534821130; Email: m.bushra@yahoo.com
How to cite this article: Mohamed A E, Moawia G, Elsafi A A, Mahmoud S B, Mohamed E, et al. The Sonographic Assessment of Kidneys in Patients with 002 Sickle Cell Disease. Curr Trends Clin Med Imaging. 2017; 1(1): 555553. DOI: 10.19080/CTCMI.2017.01.555553
Abstract
Sickle cell disease is a hereditary disorder of hemoglobin affecting several abdominal organs and still remaining a health problem. The objective of this study is to assess the renal echogenicity and volume and ascertain the correlation with the duration of disease. Materials and methods: in a cross sectional study, 77 patients with sickle cell disease in West of Sudan were studied by ultrasound from the period of February to November 2016. The patients were scanned with 3.5 MHz probe; renal length, volume and echogenicity were assessed.
Results The Sickle cell disease was common in female higher than male (42 vs. 35). The renal size and echogenicity were raised in association with sickle cell disease. The duration of the disease had significant impact on renal size, length and echogenicity, p-value = 0.00.
Conclusion Renal size and echogenicity were raised in proportion to duration of sickle cell disease. The left kidney was more enlarged and hyperechoic than the right kidney. Early sonographic assessment of renal volume and echogenicity is useful for predicting renal diseases in patients with sickle cell diseases.
Keywords: Sonographic; Assessment; Kidneys; Sickle; cell; Disease
Abbreviations: SCD: Sickle Cell Disease; Rt: Right; Lt: Left; HS: Hemoglobin S
Introduction
Sickle cell disease (SCD) is a hereditary disorder caused by the formation of abnormal hemoglobin S (HbS). It causes vasoocclusions especially the tiny blood vessels, infections, ischemias, acute pains, and fibrosis of abdominal organs [1-3]. SCD can involve several organs and systems and such complications may lead to severe mortality and morbidity. The presence of abnormal circulating RBCs lead to blockage of microcirculation that may cause ischemia and infarction [4].
The purpose of the study is to assess the kidneys in patients with SCD using gray scale ultrasonography. Ultrasound is an accurate imaging method and provides thorough assessment of kidney size, echotexture and echogenicity. SCA is associated with several functional and structural complications of the kidney [5] which may develop to chronic renal failure (CRF) and end-stage renal disease [6]. In previous studies renal failure had been reported in SCD and the prevalence ranged from 5 to 18% of the total population [7]. Kidneys are vulnerable of SCD and infarction, especially in renal medullae which causes capillary obliteration that results in papillary and medullary necrosis. Several studies reported sonographic changes in renal parenchyma and cortex, they demonstrated diffuse increased echogenicity of renal medullae in patients with SCD [8]. On the other hand, SCD has significant impact on kidney size which was significantly increased in patients with SCD [9]. So, sonographic evaluation of kidneys is very necessary since complications of SCD may progress to renal failure.
The frequency of SCD varies in Sudan. The highest frequency was reported in Western and Eastern States [10]. This present study was done in Kordufan 9western Sudan) in which high prevalence of SDS existed. In this region, HbS is a common finding especially in tribes of African descent [11]. Since the disease affects the kidneys, the study has assessed the renal echogenicity and volume as early predictors of alterations that may progress to severe complications such as renal failure.
Materials and Methods
The study was prospective cross sectional case control type in which abdominal US examination was performed for patients with known sickle cell disease and normal Sudanese population. The target population for this study defined to include Sudanese patients with sickle cell disease. Patients with sickle cell disease of duration less than 3 years were excluded in order to give an opportunity for renal changes in size and parenchyma to appear. The study was carried out in North and West Kordofan state at Kuwaiti Pediatric Hospital, Modern Health Insurance Centre in Obeyed city, and Dr. Omar A.mageed Medical Centre in El-nehood city. The study conducted from the period of August 2014 to November 2016. A designed data collection was used to collect clinical history and demographic data.
The Ultrasound Examination
Two US machines were used in the study; the first one was accuvixxg – AVXGL30-samsung -Korea and the second one was mindary DC-N6 –China. The probe used was curve linear multihertzs probe. An US gel was poured at the top of the transducer to avoid reflection of ultrasound and to maintain a good transmission of US beam inside the body. The examination began in supine posion. The para-aortic region was examined to exclude the presence of horse show kidneys. Length, width, depth and cortical thickness of the kidneys were measured. The longitudinal dimensions of the kidneys were upper pole to lower pole to represent the longest longitudinal section. Coronal or Sagittal view were also taken. The width and depth were measured in a section perpendicular to the long axis of the kidney as assessed from the longitudinal image. The transverse section was taken to pass through the hilum of the kidney. Width and depth were then measured in two orthogonal directions; renal volume was estimated from the three orthogonal measurements on the base of ellipsoid formula.
The echogenicity of the cortex for each kidney was compared with the liver in the right side and spleen in the left side to evaluate echogenicity changes.
Statistical analysis: the data were analyzed using SPSS program, version 21. Descriptive statistics was used to find the mean and frequency of the variables. Pearson correlation was applied to find correlation of renal echogenicity and volume with duration of the SCD. P-values less than 0.05 were considered to be significant.
Results
The mean age of the participants was 9.42 years. The mean length of the right kidney was 8.69 cm, and 8.98cm for the left kidney as shown in Table 1. They were longer when compared with the normal values. The volume of the right kidney was 8.19cm3, and 8.35cm3 for the left kidney. They were larger than normal values. The SCD involved male more than female (42 vs. 35) as shown in Figure 1. The duration of SCD was correlated with renal length, volume and echogenicity. There was positive correlation of duration of the disease with renal length, volume and echogenicity; r = 0.50, 0.44, 0.5, 0.50 for Rt. Kidney length, Lt. Kidney length, Rt. Kidney Volume, Lt. Kidney Volume respectively. The correlation of duration with renal echogenicity was negative and weak, r = -.18, -.15 for left and right kidney respectively (Table 1-3).
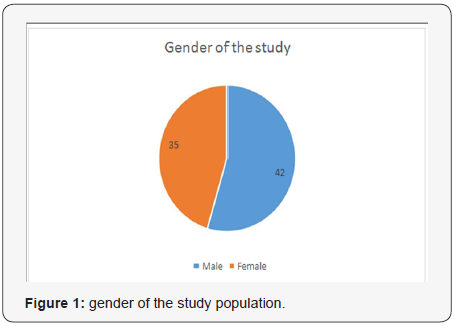
Table 1: Descriptive statistics of duration of CSD and renal measurements compared of referent values.
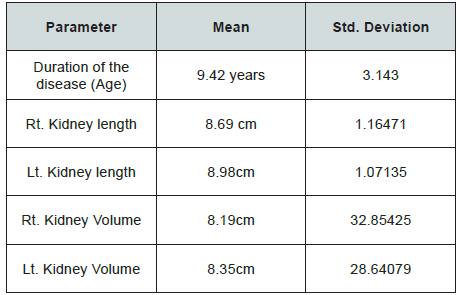
Table 2:The correlation of duration of SCD with renal length, renal volume and echogenicity.
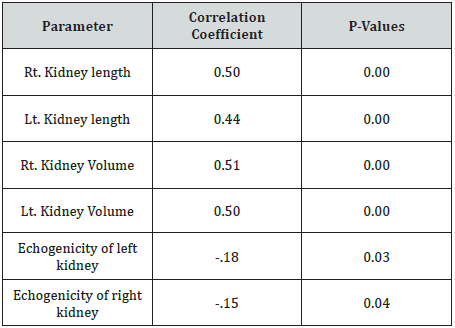
Table 3: Frequency of size and echogenicity of kidneys in patients with CSD.

Discussion
SCD is associated with several abdominal organ changes There are many diseases that alter the echogenicity and size of the kidneys due to variety of physiologic and pathologic processes [12,13]. However, to our knowledge, most of the studies assessed the songraphy of the whole abdominal organs, but few studies demonstrated the sonographic changes in one organ such as kidneys. In this study we focused on the changes that involved the size and echogenicity of the kidneys in patients with SCD. Early sonographic evaluation of kidneys helps to prevent severe complications that may end to renal failure.
In the present study, the SCD has an impact on size of the kidneys. In previous studies the size of kidneys had been evaluated in general without determining which left or right one. But in this study we have assessed the size of each kidney separately. It was observed that the left kidney is more enlarged than the right kidney. In a study conducted by Ali et al. [14] reported that the renal enlargement was 0.30.1% in SCD. This finding was agreed with our result, thus we found 32.47 % (summation of incidence of right and left renal enlargements). Accordingly, it was observed the measurement of length of the left kidney was higher than that of the right kidney. The etiology of renal enlargement in SCD is unknown. It was suggestive to glomerular hypertrophy and increased renal blood volume as likely contributors [15,16].
The effect of SCD on renal echogenicity had been reported in previous studies. The increased echogenicity involves medullae and cortex and may be diffused or focal. In the current study, the incidence of increased echogenicity (hyperechoic) was 36.66%. This result is agreed with Daneil et al. [17] who reported increased echogenicity in various types of sickle hemoglobinopathies. Our finding was also consistent with Ali et al who reported increased focal and diffused echogenicity in 7.1% and 9.5% of the cases [14]. Marina et al reported that increased echogenicity was found in17.6% of patients with sickle-cell syndromes [18]. The etiology of increased echogenicity was unknown; however, glomerulofibrosis, renal papillary necrosis, renal sclerosis, increased concentrations of iron deposits within tubular epithelial cells, focal scarring and interstitial fibrosis in vasa recta system have been suggested as contributing factors [14].
SCD is a disorder that worsens over time and so several complications had been reported. The current study showed a significant positive correlation between duration of SCD and renal length and volume (p-value = 0.00). It was observed that renal volume and length increased significantly over time due to SCD. This correlation is weak since the enlargement of kidneys occurs in early stage, but over time the complications accelerate
kidney disease progression to end-stage renal failure which was characterized by shrunken kidney [19]. This indicates that the duration of SCD had a negative impact on kidneys and this in turn lead to significant change in renal volume and echogenicity.
Conclusion
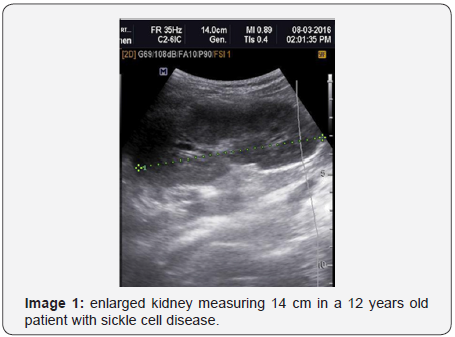
The echogenicity and volume of kidneys were raised in patients with SCD. The left kidney was more hyperechoic and most enlarged than the right kidney. Early sonographic assessment of kidneys will help to prevent and manage the renal complications of SCD (Image 1&2).

References
- Desai DV, Hiren D (2004) Sickle cell disease: History andorigin. The internet journal of haematology 2: 47.
- Lanzkowsky P (2011) Sickle cell anaemia. Manual of paediatric haematology (5th edn,). Ohio: Academic press, USA, pp. 200-231.
- Aldrich TK, Nagel RL (1998) Pulmonary complications of sickle cell disease. In: Reynolds HY, Bone RC, Dantzker DR, et al. (Eds). Pulmonary and critical care medicine (6th edn), Mosby, St. Louis, USA, pp. 1-10.
- Gael J, Lonergan Col, David B, Cline, Susan L, et al. (2001) Sickle Cell Anemia. Radio Graphics 21(4): 971-994.
- Ataga KI, Orringer EP (2000) Renal abnormalities in sickle cell disease. Am J Hematol 63(4): 205-211.
- Mapp E, Karasick S, Pollack H, Wechsler RJ, Karasick D (1987) Uroradiological manifestations of S-hemoglobinopathy. Semin Roentgenol 22(3): 186-194.
- Scheinman JI (1994) Sickle cell nephropathy. In: Pediatric Nephrology, edited by Holliday M, Barratt TM, Avner ED, Williams & Wilkins, Baltimore, USA, pp. 908-919.
- Rosado P, Paixao W, Schmitt D, Penha F, Carvalho A, Tavares; Amadora/ PT. Sickle cell anemia - a review of the imaging findings.
- Ibinaiye PO, Babadoko AA, Yusuf R, Hassan AA (2013) Renal complications of sickle cell anemia in Zaria, Nigeria: An ultrasonographic assessment. West Afr J Radiol 20(1): 19-22
- Alk RJ, Jennette JC (1994) Sickle cell nephropathy. Adv Nephrol 23: 133-147.
- Bayoumi RA, Taha TS, Saha N (1985) A study of some genetic characteristics of the Fur and Baggara tribes of the Sudan. Am J Phys Anthropol 67(4): 363-370.
- Slovis TL, Bernstein J, Gruskin A (1993) Hyperechoic kidneys in the newborn and young infant. Pediatr Nephrol 7(3): 294-302.
- Siegel MJ (2002) Urinary tract. In: Siegel MJ, Pediatric sonography. (3rd edn), Lippincott Williams & Wilkins, Philadelphia, USA, pp. 385-473.
- Ali Balcı, Sinem Karazincir, Özlem Sangün, Edip Gali, Turgay Daplan, et al. (2008) Prevalence of abdominal ultrasonographic abnormalities in patients with sickle cell disease. Diagn Interv Radiol 14(3): 136-137.
- Mapp E, Karasick S, Pollack H, Wechsler RJ, Karasick D (1987) Uroradiological manifestations of S-hemoglobinopathy. Semin Roentgenol 22(3): 186-194.
- Walker TM, Beardsall K, Thomas PW, Serjeant GR (1996) Renal length in sickle cell disease: observations from a cohort study. Clin Nephrol 46(6): 384-388.
- Daniel Zinn, Jack O, Harris L (1993) Focal and diffused echogenicity in the renal parenchyma in patients with sickle hemoglobinopathies-an Observational study. Jultrasound Med 12(4): 211-214.
- Marina G Papadaki, Antonios C Kattamis, Irene G, et al. (2003) Pediatric Radiology 33(8): 515-521.
- Abdullah Alhwiesh. Saudi J Kidney Dis T