Abstract
Multiple sclerosis (MS) is the chronic autoimmune demyelinating disease affecting the central nervous system (CNS), marked by inflammation, damage to nerve fibers (axonal injury), and progressive neurological deterioration. Traditional treatments for MS primarily aim at regulating immune responses and slowing down the disease progression; however, they often fail in effectively promoting remyelination. In recent years, exosome-based treatment strategies have emerged as a promising approach for stimulating myelin repair and neuroregeneration. Exosomes, nano-sized extracellular vesicles released by different cell types, which play critical roles in cell-to-cell communication and have been shown potential in delivering bioactive molecules such as proteins, RNAs, and lipids directly to the target cells, including oligodendrocyte precursor cells (OPCs). The present brief review examines the therapeutic potential of exosome-mediated strategies in promoting remyelination in MS, highlighting the significant preclinical findings, sources of therapeutic exosomes, mechanisms of action, and delivery challenges. Furthermore, the review also addresses the current limitations and future directions for clinical translation, offering insights on how exosome-based interventions could transform regenerative treatments for MS and other demyelinating diseases.
Keywords:Multiple Sclerosis; Myelination; Exosome; Oligodendrocyte; Autoimmune; Demyelinating Disease
Introduction
Multiple sclerosis (MS) is a chronic, immune-mediated disease of the central nervous system (CNS), characterized by demyelination, axonal damage, and persistent neuroinflammation. Globally, around 2.8 million people affected by this condition, it progressively impairs the neurological function due to the loss of myelin sheaths and failure of remyelination mechanisms (Walton et al., 2020) [1]. Current treatment strategies mainly target immunomodulation to reduce relapse rates and delay disease progression; however, these approaches have limited efficacy in restoring of myelin sheath and neurodegeneration (Loma & Heyman, 2011) [2]. Myelination is essential for the rapid conduction of nerve signal transmission and for maintaining axonal integrity. In MS, oligodendrocyte precursor cells (OPCs) often fail to mature and remyelinate the injured axons, which could be due to an unfavorable microenvironment, chronic inflammation, and inhibitory molecular signals (Franklin & Ffrench-Constant, 2017) [3]. Therefore, there is an urgent need for regenerative therapies that directly support remyelination and neuronal recovery. Exosomes are small extracellular vesicles (30–150 nm in size) secreted by different cell types, which emerged as promising therapeutic agents for CNS diseases. This is largely due to their role in mediating cell-to-cell communication and ability to transport bioactive molecules including proteins, mRNAs, miRNAs, and lipids (Raposo & Stoorvogel, 2013) [4]. Many preclinical studies have revealed that exosomes derived from sources such as mesenchymal stem cells (MSCs), neural stem cells (NSCs), and oligodendrocytes could promote remyelination by enhancing OPC differentiation, reducing inflammation, and modulating the immune response (Pusic et al., 2014; Cheng et al., 2024; Osorio-Querejeta et al., 2020) [5-7].
Despite these promising findings, a comprehensive understanding of how exosomes from different sources and molecular levels specifically contribute to remyelination in MS is warranted. Most of the existing reviews either focus broadly on the exosome roles in neurodegeneration or emphasize their use in drug delivery systems, often neglecting the targeted mechanisms of myelin repair. Moreover, translating these preclinical findings into clinical practice remains a significant hurdle due to challenges in exosome isolation, characterization, targeted delivery, and large-scale production (Lener et al., 2015) [8]. The present review uniquely focuses on exotic-based strategies specifically for enhancing remyelination in MS, rather than general neuroprotective or anti-inflammatory effects. It highlights the therapeutic potential of exosomes as endogenous modulators of myelination, drawing from the recent experimental studies and mechanistic insights. Furthermore, it identifies current limitations and proposes future research directions for optimizing exosome-mediated interventions as a novel regenerative strategy in demyelinating disorders.
Methodology
This review was conducted using a structured and narrative synthesis approach to collate and critically evaluate existing scientific literature on exosome-based myelination strategies in multiple sclerosis (MS). The methodology was designed to ensure comprehensive coverage of relevant studies, with a focus on preclinical and emerging clinical data. A systematic search was conducted across the major scientific databases including PubMed, Scopus, Web of Science, and Google Scholar for articles published between 2010 and 2024. The following keyword combinations were used “exosomes” AND “multiple sclerosis”; “exosome therapy” AND “myelination”; “extracellular vesicles” AND “remyelination” AND “CNS”; “oligodendrocyte precursor cells” AND “exosomes”; “MSC-derived exosomes” AND “neurodegeneration”.
Inclusion Criteria
• In vitro or in vivo studies focusing on the role of
exosomes in myelination or remyelination.
• Research specifically investigating MS models or
demyelinating conditions relevant to MS pathology.
• Articles examining therapeutic applications or
mechanisms of exosome-mediated myelin repair.
Exclusion criteria:
• Non-English publications, conference abstracts,
editorials, and commentaries.
• Studies unrelated to CNS myelination or not involving
exosomes as a therapeutic component.

Data Extraction and Analysis
Relevant data were extracted manually and organized into
thematic categories:
• Source and characterization of therapeutic exosomes.
• Mechanisms by which exosomes promote myelination.
• Effects on oligodendrocyte precursor cell (OPC)
differentiation and remyelination.
• Challenges in exosome delivery, targeting, and
translational potential.
The data synthesis elaborated a narrative comparative approach, highlighting the common findings, differences in methodology, and key outcomes across studies. Specific emphasis was placed on identifying the gaps in current research and proposing future directions.
Results and Discussion
Therapeutic Potential of Exosomes in Promoting Remyelination
Exosomes are originated from different cell types of mesenchymal stem cells (MSCs), neural stem cells (NSCs), and oligodendrocyte precursor cells (OPCs) which has been suggested as promising role in promoting remyelination in experimental models of multiple sclerosis (MS) (Zhang et al., 2022) [9]. These extracellular vesicles transport molecular cargo including microRNAs (miRNAs), mRNAs, proteins, and lipids that play critical roles in modulating the oligodendrocyte differentiation and CNS repair mechanism. Interestingly, preclinical studies related to young rats exposed to an enriched environment both in vitro and in vivo reported that increased levels of OPC differentiation and myelination (Pusic & Kraig., 2014) [5]. Similarly, when these exosomes were enriched with miR-2019, a vital modulator of oligodendrocyte differentiation, and their administration into the CNS promoted remyelination in demyelinated mouse models. In accordance with this, Osorio-Querejeta et al. (2020) [7] exhibited that miR-219a-5p–enriched extracellular vehicles (EVs) significantly increased the myelin regeneration in MS mouse models, suggesting that functional RNA cargo could mimic natural repair mechanisms. These interesting findings promote the efficacy of exosome-based treatments in stimulating the CNS repair, which is strongly mediated by their cellular source and molecular cargo.
Source-Dependent Efficacy of Exosomes
The therapeutic effect of exosomes is closely dependent on their cellular origin. The exosomes of the Mesenchymal Stem Cells (MSCs) have been extensively reported due to their immunomodulatory properties and safety profile (Vizoso et al., 2023) [10]. Studies reported by Liao et al. (2025) [11] suggested that MSC-derived exosomes showed significantly reduced neuroinflammation and promoted functional recovery after spinal cord injury by activating the cell survival PI3K/Akt pathway and a known regulator of myelination. Similarly, the exosomes derived from neural stem cells (NSCs) have been shown to be effective in targeting the demyelinated areas and supporting neural repair (Li & Fang., 2023) [12]. These exosomes are enriched with neurotrophic factors and signaling molecules such as brainderived neurotrophic factor (BDNF) and fibroblast growth factor 2 (FGF2), both of which support oligodendrocyte survival and maturation (Leferink & Heine 2018) [13].
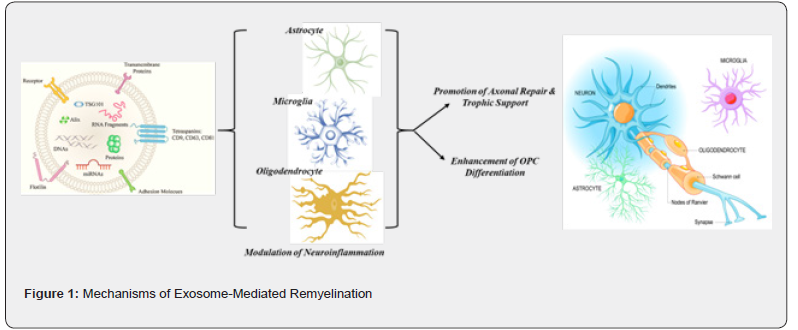
Mechanisms of Exosome-Mediated Remyelination
The exosomes are nanosized extracellular vesicles (30–150 nm), which serve as intercellular messengers, delivering complex molecular cargo including proteins, microRNAs (miRNAs), mRNAs, and lipids to the target cells (Tenchov et al., 2022) [14]. These exosomes have gained attention due to their ability to cross the blood brain barrier, and it could influence multiple cell types in the central nervous system (CNS), thereby, rendering them as highly promising therapeutic agents for remyelination in demyelinating disorders like Multiple Sclerosis (MS) (Singh et al., 2024) [15]. The main mechanism by which these exosomes regulate the targeted delivery of promylinating miRNAs including miR-219 and miR-338 could modulate the gene expression of oligodendrocyte precursor cells (OPCs) for effective remyelination (Wang et al., 2024) [16]. These miRNAs suppress the transcriptional factors like Sox6 and Hes5 that is indirectly related to myelination, thereby promoting the differentiation of OPCs into mature oligodendrocytes capable of restoring the myelin sheaths (Pusic & Kraig, 2014) [5]. In addition to this, the preclinical evidence suggests that exosomes enriched with miR-219a-5p not only significantly enhanced the myelin regeneration but also support the concept that RNA cargo plays an important role in mimicking the endogenous repair mechanisms (Osorio-Querejeta et al., 2020; Chivero et al., 2020; Dolcetti et al., 2020; Maciak et al., 2023) [7,17,18,19]. The exosomes enhance the remyelination through miRNA mediated gene regulation, and it aids in neural repair by regulating the immune response within the central nervous system.
Exosomes also exhibit potent immunomodulatory effects, which is very crucial in countering the chronic neuroinflammation that affects the remyelination in multiple sclerosis. These exosomes regulate the immune cells by downregulating the proinflammatory cytokines such as TNF-α, IL-1β and upregulating the anti-inflammatory mediators like IL-10 and TGF-β (Li et al., 2025) [20]. Furthermore, they promote the phenotypic switch of microglia from pro-inflammatory M1 phenotype to anti-inflammatory M2 phenotype (Osorio-Querejeta et al., 2020) [7], creating a permissive microenvironment that supports remyelination and neural repair (Li et al., 2025) [20]. In addition to the immune modulation, the exosomes provide trophic support to the target cells by delivering the neurotrophic factors such as brain-derived neurotrophic factor (BDNF), glial cell-derived neurotrophic factor (GDNF), nerve growth factor (NGF), and fibroblast growth factor 2 (FGF2) (Zhu et al., 2023; Yari et al., 2023) [21,22]. These molecules increase the synaptic plasticity, neuronal survival, and glial support, which is essential for efficient remyelination (Zhang et al., 2015) [23]. They also facilitate the formation of new myelin sheaths around the surviving axons for stabilizing the axon-glial interactions (Zhang et al., 2015) [23]. In addition to providing trophic support by regulating the neurotrophic factors, these exosomes also improve the remyelination by modulating the OPC recruitment, survival, and differentiation via activation of key signaling pathways and delivery of survival-promoting proteins. In accordance to this, many investigations suggested that for the efficient remyelination to occur in preclinical models, the OPCs have to be recruited to the lesion sites, making it survive in the inflammatory milieu, and differentiate into functional oligodendrocytes pertaining to myelination (Pusic & Kraig., 2015; Men et al., 2019; Lombardi et al., 2019; Wang et al., 2017) [5,24,25,20]. The exosomes promote these processes by activating key signaling pathways such as PI3K/Akt, Wnt/β-catenin, and Notch, which are vital for cell survival and differentiation. Additionally, they also carry survivalpromoting proteins like HSP70, HSP90, and chemoattractant like CXCL12, which help the OPC migration, maturation and enhance their integration into demyelinated regions (Tian et al., 2018) [26].
The demyelinating lesions in multiple sclerosis are characterized by aggressive extracellular matrix enriched with inhibitory molecules such as chondroitin sulfate proteoglycans and fibronectin; the exosomal cargo has been speculated to repair the lesions by modifying this environment. The exotics help to remodel this extracellular matrix by delivering matrix metalloproteinases (MMP-2 & MMP-9) enzymes, which degrade inhibitory substrates and facilitate OPC process extension into the lesion site (Pusic & Kraig, 2014). Further, these exosomes support the coordinated signals among the neurons and glial cells (astrocytes, microglia, and oligodendrocytes). For instance, the astrocyte-derived exosomes enriched in lipids contribute to membrane biogenesis required for the myelin formation, while neuron-derived exosomes regulate the OPC differentiation through synaptic activity (Frühbeis et al., 2012) [27]. This crosstalk among glial and neuronal populations increases the efficiency and synchronization of remyelination. In a nutshell, when taken together, these multifaceted actions highlight the central role of exosomes in organizing the myelin repair, positioning them as a highly adaptable and biologically relevant tool in restoring the CNS integrity for demyelinating diseases.
Delivery Challenges and Limitations of Exosome-Based Remyelination Therapies
The exosome-based therapies offer promising potential for remyelination in multiple sclerosis (MS), however their clinical translation faces many challenges. A major hurdle is the heterogeneity of exosomes, which differs with cellular origin, donor characteristics, and environmental conditions affecting the therapeutic consistency and efficacy (Li et al., 2025) [20]. Targeting specificity also remains restricted; while exosomes can cross the blood–brain barrier, they could also get accumulated in the off-target organs like the liver and spleen (Liang et al., 2023) [28]. The efforts in the advancement of CNS delivery by ligand modification are still in its early stages. Additionally, accessible production and purification methods are deficient, with current techniques yielding varying purity and possible contaminants could accidentally aggravate the biological responses (Kumar et al., 2020) [29]. Also, storage variability further complicates clinical use, as exosomes are very sensitive to temperature and handling conditions could compromise the cargo integrity. Similarly, safety and regulatory concerns hamper progress. Although exosomes are commonly considered low in immunogenicity, they could carry immunostimulatory molecules or tumor-promoting factors, predominantly when sourced from poorly characterized or allogeneic cells (Liu et al., 2023) [30]. The absence of clear regulatory guidelines on classification, quality control, and ethical sourcing especially from the fetal tissues further delays the approval and acceptance. Moreover, in vivo mechanisms remain incompletely understood; while studies highlight the roles of miRNA delivery and its immunomodulation, the complex interactions of exosomes in the CNS, their long-term fate, and their risk in off-target effects warrant deeper investigation. Overcoming these limitations will require interdisciplinary collaboration to improve the delivery strategies, increase safety profiling, and establish robust clinical and regulatory frameworks.
Future Directions and Translational Outlook
The future of exosome-based therapy for remyelination in multiple sclerosis (MS) is controlled by the transformative advancements and innovations in bioengineering, delivery science, and molecular biology. One of the most promising ways involves in improving the specificity and efficacy of exosome delivery through the surface modifications (Li et al., 2025) [20]. The functionalization with ligands or peptides selectively binds to the demyelinated axons or oligodendrocyte precursor cells (OPCs), which could significantly increase targeting of the central nervous system (CNS) (Jiang et al., 2022) [31]. Additionally, the exosomes by integrating with the biocompatible biomaterials such as hydrogels or nanoparticles could enable sustained and localized release, standardizing the therapeutic impact at lesion sites while minimizing systemic exposure (Ojeda-Hernández et al., 2022) [32]. Along with the advancements in the development of exosome mimetics, the synthetic nanovesicles also could offer customizable and scalable replacements with better control over cargo loading, stability, and pharmacokinetics (Sen et al., 2023) [33]. The clinical translation of exosome-based remyelination therapies in multiple sclerosis could be advanced by several strategic priorities, which must be addressed. Standardization of isolation, purification, and characterization methods is critical in ensuring consistency, while establishing quality control criteria including the particle size, surface markers, and cargo content which could increase the reproducibility and regulatory compliance (Dilsiz., 2024) [34].
More advanced research should focus on engineering the exotics for targeted delivery using ligands or surface modifications, with alternative administration routes like intranasal or intrathecal injection contributing to improved CNS access (Nieland et al., 2023) [35]. Mechanistically, identifying active cargo particularly the key microRNAs, lipids, and proteins could promote OPC differentiation will benefit from systems biology tools like transcriptomics and proteomics (Cohn et al., 2021) [36]. Preclinical studies should increasingly use humanized or non-human primate models for better understanding of MS pathophysiology, along with longitudinal evaluations of safety, biodistribution, and efficacy. Clinically, the early-phase trials are required to evaluate the safety and therapeutic potential, supported by regulatory frameworks personalized to biologic nanotherapeutics. Therefore, personalized approaches, integrating molecular profiling, and AI-driven analytics could further refine the treatment strategies and monitor the long-term outcomes efficiently. In summary, while exosome-based therapies offer enormous potential in promoting the remyelination in multiple sclerosis, their successful clinical implementation hinges on addressing several scientific, technological, and regulatory challenges. A multidisciplinary and collaborative approach could engage the molecular biologists, neurologists, bioengineers, and regulatory bodies, will be crucial for unravelling the full neurodegenerative potential of exosomes and translating them into a sustainable therapeutic modality for demyelinating disorders.
Conclusion
Exosome-based therapies signify a transformative strategy in addressing the remyelination gap of multiple sclerosis. Through targeted molecular signaling, immunomodulation, and support of OPC maturation, exosomes offer a multifaceted approach to CNS repair. While preclinical evidence is compelling, rigorous translational studies, optimized delivery systems, and standardized manufacturing protocols are crucial for their clinical application. Future research is warranted in humanized models, safety profiling, and accessible production to unlock the full potential of this regenerative modality.
References
- Walton C, King R, Rechtman L, Kaye W, Leray E, et al. (2020) Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS. Multiple Sclerosis Journal 26(14): 1816-1821.
- Loma I, Heyman R (2011) Multiple sclerosis: pathogenesis and treatment. Current neuropharmacology 9(3): 409-416.
- Franklin RJ, Ffrench-Constant C (2017) Regenerating CNS myelin—from mechanisms to experimental medicines. Nature Reviews Neuroscience 18(12): 753-769.
- Raposo G, Stoorvogel W (2013) Extracellular vesicles: exosomes, microvesicles, and friends. Journal of Cell Biology 200(4): 373-383.
- Pusic AD, Kraig RP (2014) Youth and environmental enrichment generate serum exosomes containing miR‐219 that promote CNS myelination. Glia 62(2): 284-299.
- Cheng LF, You CQ, Peng C, Ren JJ, Guo K, et al. (2024) Mesenchymal stem cell-derived exosomes as a new drug carrier for the treatment of spinal cord injury: A review. Chinese Journal of Traumatology, 27(03): 134-146.
- Osorio-Querejeta I, Carregal-Romero S, Ayerdi-Izquierdo A, Mäger I, Nash LA, et al. (2020) MiR-219a-5p enriched extracellular vesicles induce OPC differentiation and EAE improvement more efficiently than liposomes and polymeric nanoparticles. Pharmaceutics 12(2): 186.
- Lener T, Gimona M, Aigner L, Börger V, Buzas E, et al. (2015) Applying extracellular vesicles based therapeutics in clinical trials–an ISEV position paper. Journal of extracellular vesicles 4(1): 30087.
- Zhang J, Buller BA, Zhang ZG, Zhang Y, Lu M, et al. (2022) Exosomes derived from bone marrow mesenchymal stromal cells promote remyelination and reduce neuroinflammation in the demyelinating central nervous system. Experimental neurology 347: 113895.
- Vizoso FJ, Costa LA, Eiro N (2023) New era of mesenchymal stem cell-based medicine: Basis, challenges and prospects. Revista Clínica Española (English Edition) 223(10): 619-628.
- Liao Z, Zeng J, Lin A, Zou Y, Zhou Z (2025) Pre-treated Mesenchymal Stem Cell-Derived Exosomes: A New Perspective for Accelerating Spinal Cord Injury Repair. European Journal of Pharmacology 992: 177349.
- Li Y, Fang B (2023) Neural stem cell-derived extracellular vesicles: the light of central nervous system diseases. Biomedicine & Pharmacotherapy 165: 115092.
- Leferink PS, Heine VM (2018) The healthy and diseased microenvironments regulate oligodendrocyte properties: implications for regenerative medicine. The American Journal of Pathology 188(1): 39-52.
- Tenchov R, Sasso JM, Wang X, Liaw WS, Chen CA, et al. (2022) Exosomes─ nature’s lipid nanoparticles, a rising star in drug delivery and diagnostics. ACS nano 16(11): 17802-17846.
- Singh G, Mehra A, Arora S, Gugulothu D, Vora LK, et al. (2024) Exosome-mediated delivery and regulation in neurological disease progression. International Journal of Biological Macromolecules 264(Pt 2): 130728.
- Wang H, Moyano AL, Ma Z, Deng Y, Lin Y, et al. (2017) miR-219 cooperates with miR-338 in myelination and promotes myelin repair in the CNS. Developmental cell 40(6): 566-582.
- Chivero ET, Liao K, Niu F, Tripathi A, Tian C, et al. (2020) Engineered extracellular vesicles loaded with miR-124 attenuate cocaine-mediated activation of microglia. Frontiers in Cell and Developmental Biology 8: 573.
- Dolcetti E, Bruno A, Guadalupi L, Rizzo FR, Musella A, et al. (2020) Emerging role of extracellular vesicles in the pathophysiology of multiple sclerosis. International journal of molecular sciences 21(19): 7336.
- Maciak K, Dziedzic A, Saluk J (2023) Remyelination in multiple sclerosis from the miRNA perspective. Frontiers in Molecular Neuroscience 16: 1199313.
- Li J, Wang J, Chen Z (2025) Emerging role of exosomes in cancer therapy: progress and challenges. Molecular Cancer 24(1): 13.
- Zhu ZH, Jia F, Ahmed W, Zhang GL, Wang H, et al. (2023) Neural stem cell-derived exosome as a nano-sized carrier for BDNF delivery to a rat model of ischemic stroke. Neural Regeneration Research 18(2): 404-409.
- Yari H, Mikhailova MV, Mardasi M, Jafarzadehgharehziaaddin M, Shahrokh S, et al. (2022) Emerging role of mesenchymal stromal cells (MSCs)-derived exosome in neurodegeneration-associated conditions: a groundbreaking cell-free approach. Stem cell research & therapy 13(1): 423.
- Zhang Y, Chopp M, Meng Y, Katakowski M, Xin H, et al. (2015) Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. Journal of neurosurgery 122(4): 856-867.
- Men Y, Yelick J, Jin S, Tian Y, Chiang MSR, et al. (2019) Exosome reporter mice reveal the involvement of exosomes in mediating neuron to astroglia communication in the CNS. Nature communications 10(1): 4136.
- Lombardi M, Parolisi R, Scaroni F, Bonfanti E, Gualerzi A, et al. (2019) Detrimental and protective action of microglial extracellular vesicles on myelin lesions: astrocyte involvement in remyelination failure. Acta neuropathologica 138(6): 987-1012.
- Tian Y, Yin H, Deng X, Tang B, Ren X, et al. (2018) CXCL12 induces migration of oligodendrocyte precursor cells through the CXCR4 activated MEK/ERK and PI3K/AKT pathways. Molecular medicine reports 18(5): 4374-4380.
- Frühbeis C, Fröhlich D, Krämer-Albers EM (2012) Emerging roles of exosomes in neuronglia communication. Frontiers in physiology 3: 119.
- Liang Y, Iqbal Z, Lu J, Wang J, Zhang H, et al. (2023) Cell-derived nanovesicle-mediated drug delivery to the brain: principles and strategies for vesicle engineering. Molecular Therapy 31(5): 1207-1224.
- Kumar A, Zhou L, Zhi K, Raji B, Pernell S, et al. (2020) Challenges in biomaterial-based drug delivery approach for the treatment of neurodegenerative diseases: opportunities for extracellular vesicles. International Journal of Molecular Sciences 22(1): 138.
- Li C, Xia C, Xia C (2023) Biology and function of exosomes in tumor immunotherapy. Biomedicine & Pharmacotherapy 169: 115853.
- Jiang Y, Wang F, Wang K, Zhong Y, Wei X, et al. (2022) Engineered exosomes: a promising drug delivery strategy for brain diseases. Current Medicinal Chemistry 29(17): 3111-3124.
- Ojeda-Hernández DD, Hernández-Sapiéns MA, Reza-Zaldívar EE, Canales-Aguirre A, Matías-Guiu JA, et al. (2022) Exosomes and biomaterials: in search of a new therapeutic strategy for multiple sclerosis. Life 12(9): 1417.
- Sen S, Xavier J, Kumar N, Ahmad MZ, Ranjan OP (2023) Exosomes as natural nanocarrier-based drug delivery system: recent insights and future perspectives. 3 Biotech 13(3): 101.
- Dilsiz N (2024) A comprehensive review on recent advances in exosome isolation and characterization: Toward clinical applications. Translational oncology 50: 102121.
- Nieland L, Mahjoum S, Grandell E, Breyne K, Breakefield XO (2023) Engineered EVs designed to target diseases of the CNS. Journal of Controlled Release 356: 493-506.
- Cohn W, Melnik M, Huang C, Teter B, Chandra S, et al. (2021) Multi-omics analysis of microglial extracellular vesicles from human Alzheimer’s disease brain tissue reveals disease-associated signatures. Frontiers in Pharmacology 12: 766082.
- Li X, Corbett AL, Taatizadeh E, Tasnim N, Little JP, et al. (2019) Challenges and opportunities in exosome research—Perspectives from biology, engineering, and cancer therapy. APL bioengineering 3(1): 011503.
- Lin M, Alimerzaloo F, Wang X, Alhalabi O, Krieg SM, et al. (2025) Harnessing stem cell-derived exosomes: a promising cell-free approach for spinal cord injury. Stem Cell Research & Therapy 16(1): 182.






























