Synthesis, Structure and Magnetocaloric Properties of La0.8K0.15Li0.05MnO3 Perovskite Manganite
Y Regaieg1,2*, W Cheikhrouhou-Koubaa1, L Sicard3, M Koubaa1,4, E K Hlil5 and A Cheikhrouhou1
1LT2S lab, Digital Research Center of Sfax, Sfax Technoparc, 3021 Sfax, Tunisia
2Faculty of Sciences of Gafsa, University of Gafsa, 2112 Gafsa, Tunisia
3ITODYS, Université Paris Diderot, UMR 7086 CNRS, 75205 Paris Cedex 13, France
4Institut Supérieur de Biotechnologie de Sfax, University of Sfax, 3000 Sfax, Tunisia
5Institut Néel, CNRS, Université Grenoble Alpes, 38042 Grenoble cedex 9, France
Submission: Febraury 27, 2023; Published: March 17, 2023
*Corresponding author: Yassine Regaieg, Digital Research Center of Sfax, Sfax Technoparc, 3021 Sfax, Tunisia
How to cite this article: Y Regaieg*, W Cheikhrouhou-Koubaa, L Sicard, M Koubaa, E K Hlil and A Cheikhrouhou. Synthesis, Structure and Magnetocaloric Properties of La0.8K0.15Li0.05MnO3 Perovskite Manganite. Curr Trends Biomedical Eng & Biosci. 2024; 21(3): 556061. DOI:10.19080/CTBEB.2024.21.556061
Abstract
The synthesis, structural characterization, and magnetocaloric properties of La0.8K0.15-Li0.05MnO3 perovskite manganite are reported. Our compound was synthesized using the sol-gel technique at low temperature. The Rietveld refinement of the X-ray powder diffraction shows that La0.8K0.15-Li0.05MnO3 sample is single phase and crystallizes in a rhombohedral system with 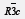 space group. Paramagnetic-ferromagnetic phase transition at TC = 231.2 K is observed for our studied compound. Magnetic entropy change, −ΔSM, deduced from isothermal magnetization curves, is about 2.39 J kg-1 K-1 in a magnetic field change of 5 T. The relative cooling power, RCP(S), value at 5 T reaches 272.4 J kg-1. This value corresponds to about 66% of that observed in pure gadolinium.
space group. Paramagnetic-ferromagnetic phase transition at TC = 231.2 K is observed for our studied compound. Magnetic entropy change, −ΔSM, deduced from isothermal magnetization curves, is about 2.39 J kg-1 K-1 in a magnetic field change of 5 T. The relative cooling power, RCP(S), value at 5 T reaches 272.4 J kg-1. This value corresponds to about 66% of that observed in pure gadolinium.
Keywords: Manganites; Sol-gel Technique; X-ray Diffraction; Isothermal Magnetization; Magnetocaloric Effect; Stoichiometric
Introduction
Magnetic refrigeration (MR) based on the magnetocaloric effect (MCE) is a very promising technology to replace the traditional gas-compression refrigeration technology[1,2]. The MCE is an intrinsic property of magnetic materials and defined as the important change of temperature of an adiabatically isolated system with the application and removal of an external magnetic field [3-5]. The MCE is characterized by the magnetic entropy change, −ΔSM, and the adiabatic temperature change, adTΔ, under magnetic field changes. Mixed valence perovskite manganites with general formula Ln1-xXxMnO3, where Ln is a trivalent rare-earth ion and X a monovalent alkaline or divalent alkaline-earth ion, are typical MCE materials and have been extensively studied these last years in view of their fascinating properties arising from spin-balance sensitivity, orbital contribution, lattice distortion, charge degrees of freedom [6,7]. Monovalent substituted perovskite manganites which are known to introduce large potential fluctuations are typical materials that have been reported [8-10]. Among the evaluated manganites, a family of Ln1-xXxMnO3 appear as very promising candidates for the desired application. In this context, the main objective of our work is to enhance the MCE of La0.8K0.2MnO3 with Li substitution. Therefore, in this pa per, we investigate the structural, magnetic and magnetocaloric properties of La0.8K0.15Li0.05MnO3 compound synthesized using the sol-gel technique at low temperature.
Introduction
Magnetic refrigeration (MR) based on the magnetocaloric effect (MCE) is a very promising technology to replace the traditional gas-compression refrigeration technology[1,2]. The MCE is an intrinsic property of magnetic materials and defined as the important change of temperature of an adiabatically isolated system with the application and removal of an external magnetic field [3-5]. The MCE is characterized by the magnetic entropy change, −ΔSM, and the adiabatic temperature change, adTΔ, under magnetic field changes. Mixed valence perovskite manganites with general formula Ln1-xXxMnO3, where Ln is a trivalent rare-earth ion and X a monovalent alkaline or divalent alkaline-earth ion, are typical MCE materials and have been extensively studied these last years in view of their fascinating properties arising from spin-balance sensitivity, orbital contribution, lattice distortion, charge degrees of freedom [6,7]. Monovalent substituted perovskite manganites which are known to introduce large potential fluctuations are typical materials that have been reported [8-10]. Among the evaluated manganites, a family of Ln1-xXxMnO3 appear as very promising candidates for the desired application. In this context, the main objective of our work is to enhance the MCE of La0.8K0.2MnO3 with Li substitution. Therefore, in this pa per, we investigate the structural, magnetic and magnetocaloric properties of La0.8K0.15Li0.05MnO3 compound synthesized using the sol-gel technique at low temperature.
Experimental Techniques
La0.8K0.15Li0.05MnO3 sample were prepared by the sol-gel technique (Pechini method) at low temperature [11]. The stoichiometric amounts of La2O3, K2CO3, Li2CO3 and MnO2 with high purity (Sigma Aldrich 99.9%) were dissolved in concentrated nitric acid to transform them into nitrates. This step was carried out at 60°C in order to accelerate the dissolution. After total dissolution, citric acid, a compliant agent, and ethylene glycol, a polymerization agent, were added. This solution is slowly evaporated at 130 °C until the formation of a residue of high viscosity. A transparent gel is developed during the heating process. The temperature was subsequently raised at 10°C min-1 rate up to 300°C to assure the propagation of a combustion, which transforms the gel into a fine powder. The resulting powder is heated at 450°C during 6 hours in order to decompose the organics. After crushing, the sample was calcined at 650°C during 12 hours and then at 800°C for the same duration with intermediate grinding. Finally, the powder was then pressed into pellets (of about 1 mm thickness and 10 mm diameter) and sintered at 900°C during 24 hours. All annealings are performed in air.
The crystallographic structure was determined by powder X-ray diffraction (XRD) at room temperature using a Panalytical diffractometer (Empyrean model). Structural analysis was carried out using the standard Rietveld technique [12]. The microstructure was studied by Scanning Electron Microscopy (SEM) using a Supra40 ZEISS FEG-SEM microscope operating at 10 kV. Magnetization measurements versus temperature in the range of 5-400K and versus magnetic applied field in the 0-5 T range were carried out using a MPMS-XL Quantum Design SQUID magnetometer. The magnetocaloric effect (MCE) was determined from the magnetization measurements versus magnetic applied field at several temperatures.
Results and Discussion
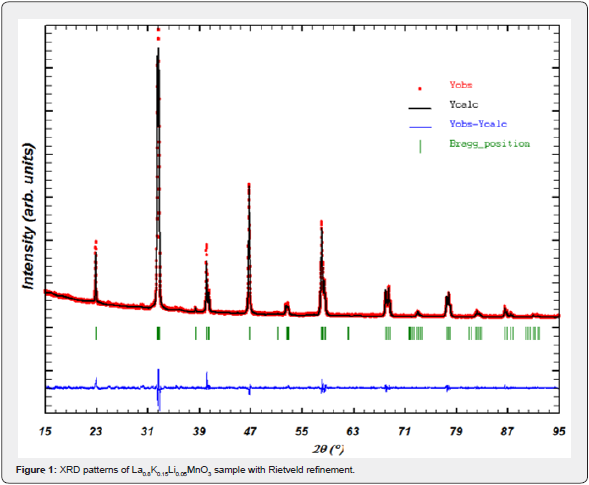
The powder X-ray diffraction pattern of the La0.8K0.15Li0.05MnO3 compound synthesized, recorded at room temperature, along with Rietveld refinement is shown in (Figure 1). The diffraction peaks can be indexed in the rhombohedral system with R3c space group. The quality factors indicating the agreement between the observed and the calculated profiles are RB = 2.58, RP = 16.1 and χ2 = 3.13. The corresponding lattice parameters and unit cell volume are a = b = 5.511(2) Å, c = 13.378(4) Å and V = 351.90 Å3. We have also calculated the Mn-O bond length and the Mn-O-Mn bond angle from the position of the ions in the unit cell and lattice parameters, which are found to be 1.961(4) Å and 164.14(3)° respectively. A SEM image of the La0.8K0.15Li0.05MnO3 sample is shown in (Figure 2). The almost polygonal grains have an average size in the submicrometer range.
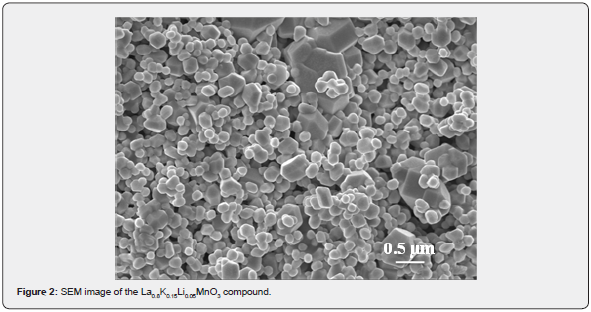
Magnetization measurements as a function of temperature, M(T), were plotted in the 5-400 K range for La0.8K0.15Li0.05MnO3 (0≤x≤0.075) compound by applying a magnetic field of 50 mT (Figure 3). Our sample exhibited a paramagnetic to ferromagnetic transition with decreasing temperature. The Curie temperature, TC (defined as the temperature at which dM/dT shows a minimum), is found to be 231.2 K.
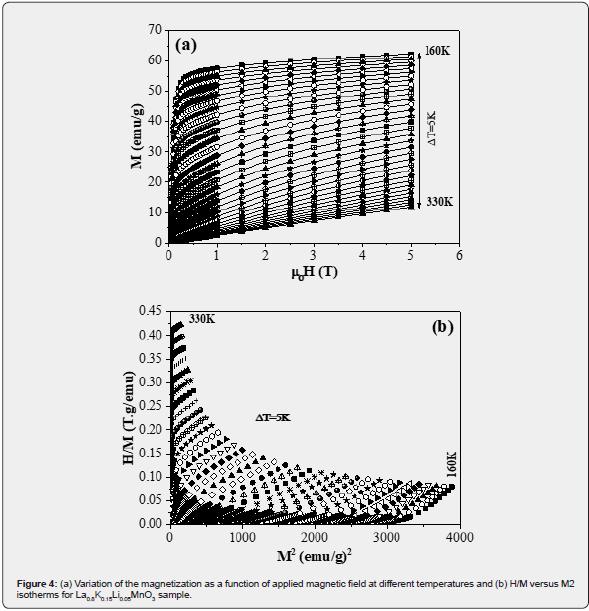
The Curie-Weiss analysis of the data above TC resulted in effective
paramagnetic moment 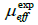 of 4.91 μB higher than the theoretical
value
of 4.91 μB higher than the theoretical
value 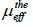 = 4.52 μB.
= 4.52 μB.
The magnetization versus magnetic field, M(H), up to 5 T at different temperatures in the 160-330 K range was also measured for La0.8K0.15Li0.05MnO3 sample (Figure4(a)). Below TC, the magnetization increases sharply below 0.5 T and tends to saturation for higher magnetic fields, which confirms the ferromagnetic behavior of our synthesized sample at low temperatures.
We plot in (Figure4(b))the Arrott curves, H/M versus M2, deduced from M(H) for La0.8K0.15Li0.05MnO3 sample. The plots show a positive slope, above TC, which indicates that a second order ferromagnetic to paramagnetic phase transition occurs [13]. Furthermore, the TC values deduced from these plots are very close to those determined from M(T).
The magnetic entropy change, −ΔSM , induced by the magnetic field change, ΔH, was determined using the M(H) curves. According to the classical thermodynamic theory based on Maxwell relations, −ΔSM , can be evaluated through the formula:
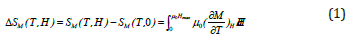
where μ0Hmax is the maximal value of the magnetic applied field. In practice the relation is approximated as:
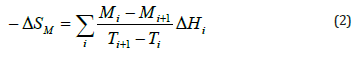
where Mi and Mi+1 are the experimental values of magnetization
measured at temperatures Ti and Ti+1 respectively, under
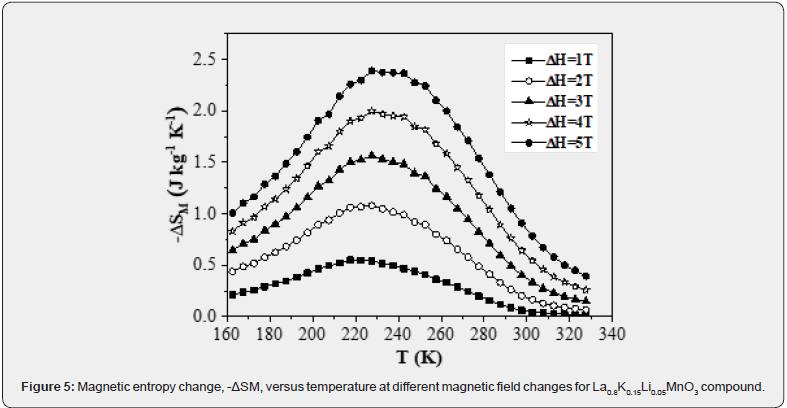
magnetic applied field Hi [14, 15]. (Figure 5) shows the temperature
dependence of the -ΔSM under different magnetic field change
for La0.8K0.15Li0.05MnO3 sample.  increases with increasing
magnetic applied field change. The maximum values of the magnetic
entropy change
increases with increasing
magnetic applied field change. The maximum values of the magnetic
entropy change  observed around TC are equal to 0.55,
1.08, 1.56, 1.99 and 2.39 J kg-1 K-1 for magnetic field changes of 1,
2, 3, 4 and 5 T respectively.
observed around TC are equal to 0.55,
1.08, 1.56, 1.99 and 2.39 J kg-1 K-1 for magnetic field changes of 1,
2, 3, 4 and 5 T respectively.
The variation of the maximum of the magnetic entropy change as a function of the magnetic field change, for the La0.8K0. 15Li0.05MnO3 sample, exhibits a monotone increase, as shown in (Figure 6(a)), which corresponds to the magnetic transition from ferromagnetic to paramagnetic states. In accordance to Oesterreicher et al. [16], the field dependence of the magnetic entropy change at TC of materials with a second order phase transition can be described by a power law of the type [17]:
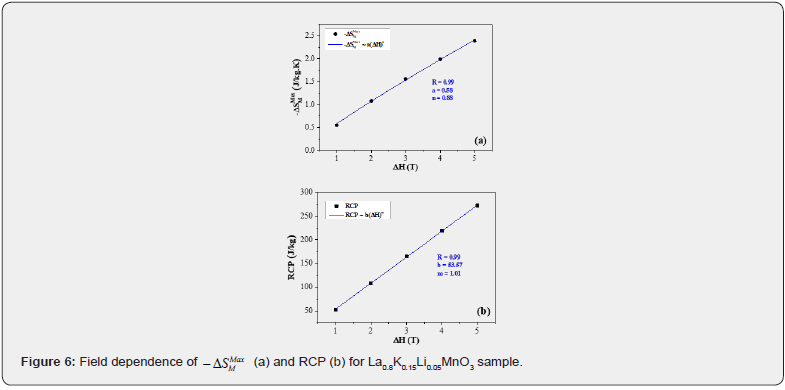
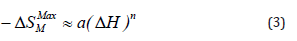
where a is a constant and the exponent n depends up on the magnetic state of the sample. The obtained value of n is 0.88, which is significantly different than 2/3, as predicted by the mean field model [17]. The deviation from n = 2/3 is due to the presence of local magnetic inhomogeneities in the vicinity of transition temperature [18]. This result is similar to those obtained for other manganites system [19-21].
In magnetic refrigeration technology, it is important that
the magnetocaloric effect extends over a large temperature
range. The relative cooling power (RCP(S)) is evaluated as
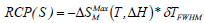 where δTFWHM is the full-width
at half-maximum of
where δTFWHM is the full-width
at half-maximum of 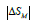 versus temperature curve [22]. We plot
in (Figure 6(b)) the RCP values as a function of the magnetic field
change, ΔH. It can be observed that the RCP values increase with
ΔH indicating that RCP is strong field dependent. Indeed, RCP can
be approximately expressed as a power law:
versus temperature curve [22]. We plot
in (Figure 6(b)) the RCP values as a function of the magnetic field
change, ΔH. It can be observed that the RCP values increase with
ΔH indicating that RCP is strong field dependent. Indeed, RCP can
be approximately expressed as a power law:

where m is the critical exponent of the magnetic transition. RCP should scale with field as a power law with an exponent m. The fitting value of m is found to be 1.01 for our sample. This m result is comparable with values previous studies [21,23,24]. (Table 1) shows the obtained results in comparison with those of several magnetic materials that could be used as active refrigerants taken from the literature. It is clear from the table that the obtained values are larger than the parent compound La0.8K0.15Li0.05MnO3 [8]. On the other hand, our RCP(S) represents about 66% of the prototype magnetic refrigerant material Gd RCP value (410 J/kg [28]) for a magnetic field change of 5T.
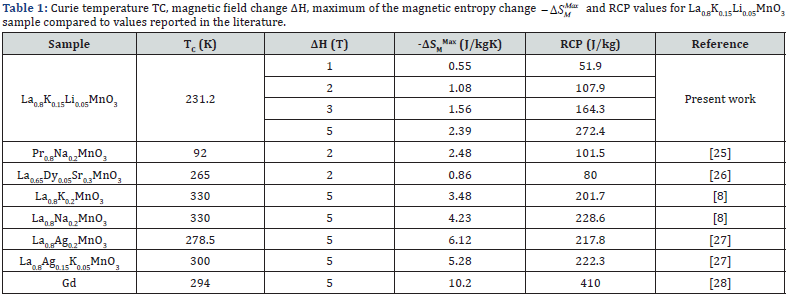
Conclusion
In summary, the polycrystalline La0.8K0.15Li0.05MnO3 sample
was prepared by the sol-gel technique at low temperature. Our
compound crystallizes in the rhombohedral system with R3c
space group. Magnetic measurements revealed that our sample
manganite undergoes a second order magnetic transition with
the paramagnetic-ferromagnetic transition at a TC ∼ 231.2 K. The
maximum of the magnetic entropy change,
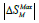 , and the relative
cooling power, RCP(S), associated with the transition are
found to be 2.39 J kg-1 K-1 and 272.4 J kg-1 respectively, under a
magnetic field change of 5T These results suggest that our synthesized
sample could be used as an active magnetic refrigerant
working below room temperature..
, and the relative
cooling power, RCP(S), associated with the transition are
found to be 2.39 J kg-1 K-1 and 272.4 J kg-1 respectively, under a
magnetic field change of 5T These results suggest that our synthesized
sample could be used as an active magnetic refrigerant
working below room temperature..
Acknowledgment
This study has been supported by the Tunisian Ministry of Higher Education and Scientific Research.
References
- Glanz J (1998) Making a Bigger Chill With Magnets, Science 279(5359): 2045.
- Zimm C, Jastrab A, Sternberg A, Pecharsky VK, Gschneidner KA, et al. (1998)Description and Performance of a Near-Room Temperature Magnetic Refrigerator. Adv Cryog Eng 43: 1759-1766.
- Casanova F, Batlle X, Labarta A (2002) Scaling of the entropy change at the magnetoelastic transition in Gd5(SixGe1-x)4. Phys Rev B 66: 212402-212405.
- Zhang XY, Chen Y, Li ZY (2007) Large magnetocaloric effect in chromium dioxide with second-order phase transition. J Phys D Appl Phys 40: 3243.
- Tishin AM, Spichkin YI (2013) The Magnetocaloric Effect and its Applications. Institute of Physics Publishing.
- Al-Yahmadi IZ, Gismelseed AM, Al Ma Mari F, Al-Rawas AD, Al-Harthi SH, et al. (2021) Structural, magnetic and magnetocaloric effect studies of Nd6Sr0.4AxMn1-xO3 (A=Co, Ni, Zn) perovskite manganites. J Alloys Comp 875: 159977.
- Regaieg Y, Ayadi F, Monnier J, Reguer S, Koubaa M, et al. (2014)Magnetocaloric properties of La67Ca0.33MnO3 produced by reactive spark plasma sintering and by conventional ceramic route. Mater Res Express 1: 046105-046118.
- Regaieg Y, Koubaa M, Cheikhrouhou Koubaa W, Cheikhrouhou A, Mhiri T (2010) Magnetocaloric effect above room temperature in the K-doped La8Na0.2-xKxMnO3 manganites. J Alloys Compd 502: 270-274.
- Ben Rejeb M, Ben Osman C, Regaieg Y, Marzouki-Ajmi A, Cheikhrouhou-Koubaa W, et al. (2017)A comparative study of La65Ca0.2(Na0.5K0.5)0.15MnO3 compound synthesized by solid-state and sol-gel process. J Alloys Comp 695: 2597-2604.
- Phan MH, Yu SC (2007) Review of the magnetocaloric effect in manganite materials. J Magn Magn Mater 308: 325-340.
- Ayadi F, Cheikhrouhou-Koubaa W, Koubaa M, Nowak S, Sicard L, et al. (2014)Effect of synthesis method on structural, magnetic and magnetocaloric properties of La7Sr0.2Ag0.1MnO3manganite. Mater Chem Phys 145: 56-59.
- Rietveld HM (1969) A profile refinement method for nuclear and magnetic structures. J Appl Crystallogr 2: 65-71.
- Banerjee SK (1964) On a generalised approach to first and second order magnetic transitions. Phys Lett 12: 16-17.
- Dinesen AR, Linderoth S, Moerup S (2005) Direct and indirect measurement of the magnetocaloric effect in La67Ca0.33−xSrxMnO3 ± δ(x∈ [0: 0.33]). J Phys Condens Matter 17: 6257-6269.
- McMichael RD, Ritter JJ, Shull RD (1993) Enhanced magnetocaloric effect in Gd3Ga5−xFexO12. J Appl Phys 73: 6946-6948.
- Oesterreicher H, Parker FT (1984) Magnetic cooling near Curie temperatures above 300 K. J Appl Phys 55: 4334.
- Franco V, Conde A (2010) Scaling laws for the magnetocaloric effect in second order phase transitions: From physics to applications for the characterization of materials. Int J Refrig 33: 465-473.
- Franco V, Bl´azquez JS, Conde A(2006) The influence of Co addition on the magnetocaloric effect of Nanoperm-type amorphous alloys. J Appl Phys 100: 064307.
- Reshmi CP, Savitha Pillai S, Suresh KG, Varma MR (2013) Room temperature magnetocaloric properties of Ni substituted La67Sr0.33MnO3. Solid State Sci 19: 130-135.
- Phan TL, Zhang P, Thanh TD, Yu SC (2014) Crossover from first-order to second-order phase transitions and magnetocaloric effect in La7Ca0.3Mn0.91Ni0.09O3. J Appl Phys115: 17A912.
- Kharrat N, Sfifir Debbebi I, Cheikhrouhou-Koubaa W, Koubaa M, Cheikhrouhou A, et al .(2019)Magnetocaloric Effect in La67Ba0.33Mn0.95Ni0.05O3 Manganite Near Room Temperature. J Supercond Nov Magn 32: 1241-1251.
- Gschneidner KA, Pecharsky VK(2000) Magnetocaloric Materials. Annu Rev Mater Sci 30: 387-429.
- Ezaami A, Sellami-Jmal E, Cheikhrouhou-Koubaa W, Koubaa M, Cheikhrouhou A (2017) Phenomenological model of magnetocaloric effect in La7Ca0.2Ba0.1MnO3 manganite around room temperature. J Supercond Nov Magn 30: 911-916.
- Ben Khlifa H, M’nassri R, Cheikhrouhou-Koubaa W, Schmerber G, Leuvrey C, et al. (2017)Structural characterization and magnetic field dependence of the magnetocaloric properties in Pr8Na0.05K0.15MnO3 ceramic. J Magn Magn Mater 439: 148-155.
- Ben Khlifa H, Regaieg Y, Cheikhrouhou-Koubaa W, Koubaa M, Cheikhrouhou A (2015) Structural magnetic and magnetocaloric properties of K-doped Pr8Na0.2-xKxMnO3 manganites. J Alloy Compd 650: 676-683.
- Felhi R, Koubaa M, Cheikhrouhou-Koubaa W, Cheikhrouhou A (2017) Structural, magnetic, magnetocaloric and critical behavior investigations of La65Dy0.05Sr0.3MnO3 manganite. J Alloys Compd 726: 1236-1245.
- Regaieg Y, Koubaa M, Cheikhrouhou-Koubaa W, Cheikhrouhou A, Sicard L, et al. (2012) Structure and magnetocaloric properties of La8Ag0.2-xKxMnO3 perovskite manganites. Mater Chem Phys 132: 839-845.
- Gschneider KA, Pecharsky VK, Tsokol AO (2005) Recent developments in magnetocaloric materials. Rep Prog Phys 68: 1479-1539.






























