Cellular Microbiology: Indispensable Tool to Dissect Host Pathogen Interaction
Juan Manuel Diaz1*, Pravil Pokharel2 and Alma Lilian Guerrero-Barrera3
1 Medical Didactic Unit, Autonomous University of Aguascalientes, Mexico
2 Institut National de Recherche Scientifique (INRS)-Centre Armand-Frappier Sante Biotechnologie, Laval, Canada
3 Morphology Department, Autonomous University of Aguascalientes, Mexico
Submission: June 14, 2022; Published: June 30, 2022
*Corresponding author: Juan Manuel Diaz, Medical Didactic Unit, Autonomous University of Aguascalientes, Aguascalientes,20131, Mexico Curr Trends
How to cite this article: Juan M D, Pravil P. Cellular Microbiology: Indispensable Tool to Dissect Host Pathogen Interaction. Curr Trends Biomedical Eng & Biosci. 2022; 21(1): 556051. DOI: 10.19080/CTBEB.2022.21.556051
Abstract
The threat of the spread of new or old infectious diseases are always a concern for human civilization. The emergence of coronavirus disease (COVID-19) and its dissemination around the world has demonstrated the risk to human health and the economy. So, it is essential to study a complete understanding of the host-pathogen interaction for the development of the disease. The field of cellular microbiology can help in the identification and characterization of different virulence factors produced by pathogens during each step of the infection process by combining techniques and approaches of classic cell biology and microbiology. Currently, the dynamic interaction of the host-pathogen relationship has been enhanced due to the cell culture, in vivo methods, in addition to microbial genomics, bioinformatics techniques, and in silico prediction methods to understand these diseases for the development of prevention methods, diagnosis and treatment worldwide.
Keywords: Cellular Microbiology; Virulence Factor; Host; Pathogen; Toxin; Infectious diseases
Introduction
Infectious diseases have been documented throughout human history since the ancient description of outbreak of infectious diseases like smallpox, tuberculosis, diphtheria, black fever, and leprosy by ancient civilization, until our days with the emerging diseases including HIV, MERS, COVID-19, ZIKA, Escherichia coli outbreaks, Influenza, etc. Although, these diseases are generated by different microorganisms, the common factor is the morbidity and mortality that these had generated individually worldwide and their effects on the economic, social, and political outcome which often lasted for centuries. With the reemergence of some important infectious diseases and its burning public health issues, the medical community started to study the origins of emerging infectious threats and its detection, response, and the future prediction to avoid havoc on human communities. So, to understand these diseases, cellular microbiology provides new technologies and paradigm to decipher at the molecular level how infectious disease develop. The basis of cellular microbiology field dated since the first mention of the word “cell” by Robert Hook in his Micrographia book in 1665, as well as the description of the first microorganism named Animalcules in 1676 by Antonie Van Leeuwenhoek. The history continued at the hands of historical scientist as Theodor Schwann, Mathias Scheilden and Rudolf Virchow with their Cell Theory, furthermore, the contribution of Louis Pasteur and Robert Koch for the discovery of the connection between microbes and infectious diseases made foundation to understand host-pathogen relation [1]. But it was not until 1996 that the term cellular microbiology was coined [2]. This new term arises due to the cross-feeding of microbiology and cellular biology to cope complex nature of study of host pathogen interaction.
The objective of cellular microbiology is to describe the mechanism and process in which the microorganism develops a homeostatic dysregulation on the host cell, that could lead in a disease. To elucidate this phenomenon cellular microbiology uses techniques like microscopy, cell culture, proteomics, genomics, immunoassays, biophysical and biochemical methods, as well as some in silico methodologies as molecular docking etc. Thus, these methods facilitate the pathophysiology study of the infectious diseases to know in depth each of the parties involved in this phenomenon: the pathogen and the host.
Pathogens
The microorganisms (bacteria, protozoa, virus, fungi, animal parasites, etc.) that can cause disease are known as pathogens. An infectious disease is developed by the entry, colonization, invasion, surveillance of the pathogen, and the consecutive pathophysiological alteration in the host. Infectious disease can have symptoms in the patients, and it will spread directly or indirectly between member of the same species or among other species. Sometime the member of species who passes the germ may have no symptoms of the disease, but simply be the carrier. Pathogenic microorganism can have access to the body through respiratory, gastrointestinal, and urogenital tract, as well as can gain ingress through skin surface. An important factor for the success of the infection is the presence and expression of virulence factors by the pathogen.
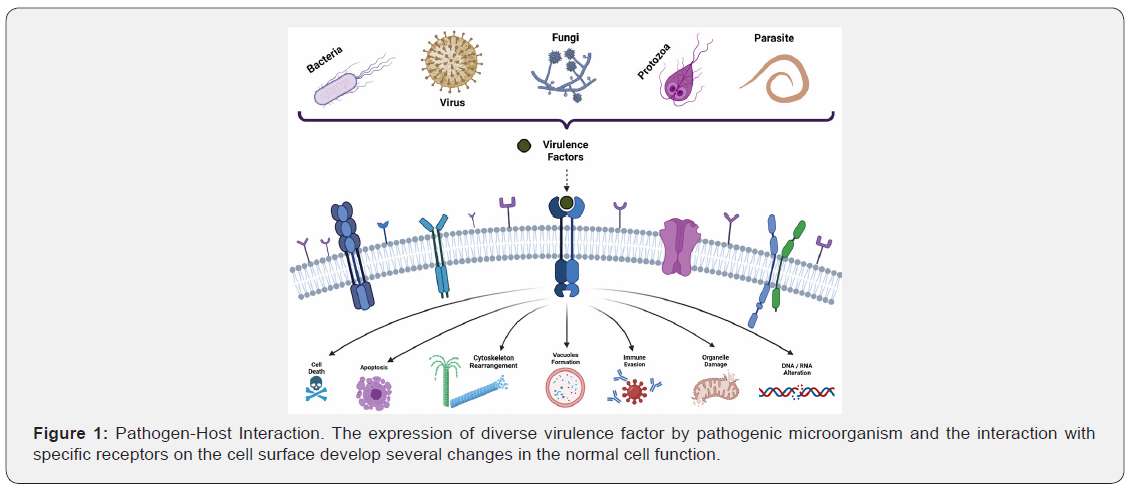
Virulence Factors (VF) are products or strategies used by pathogenic microorganism to enhance the ability of pathogens to survive in the host and/or cause infection (Figure 1). These VFs can be grouped into the following major classes: adhesins, membrane or enveloped molecules to prevent phagocytosis or serum resistance, toxins, exoenzymes, immune evasion molecules, nutrients acquisition systems, modulins and biofilm formation [3-5]. The interaction of VF with the host cell could generate a damage in the normal host function, related to the infectious process. The VF could be found genetically, inserted in the chromosomal or as a mobile genetic element that could be shared by vertical or horizontal transmission through transformation, transduction, or conjugation. These transfers can occur within a species or also between the members of different species or even different genera, generating an adaptative advantage to next generation [6,7]. The expression of the VF by the pathogen could be constitutive or regulated, this regulation is mediated by environmental conditions such as pH, osmolarity, microbial stress, nutrients availability or oxygen concentration [8].
Host Response
The interaction between host and pathogen is complex, each acting back and forth, to influence the activities and functions of the other. Development of infectious disease is governed by multiple host and pathogen VFs. Years of research and use of different animal and cellular models have elucidated some of the common pathogenic mechanisms. Those mechanisms include adherence to host cells for colonization, motility, toxin production, acquisition of essential metals and other micronutrients, evasion of host’s innate and adaptive immune defenses, dissemination within the host and to other hosts (Figure 1) [9].
Innate system of host is always on guard and works to fight off pathogens before they can start an active infection. The conserved molecules from the pathogens or VF will be identified by the receptors present on the host cell surface by the Pattern Recognition Receptors (PRRs) or by another receptor on the cell which will trigger innate and adaptive immune system [10]. These receptors could be used by the microorganism to attach on cell surface and initiate the infection following the principles of ligand-receptor interaction (Figure 1). After this interaction, the host could develop morphological changes and tissues disorganization by polymerization and depolymerization of the cytoskeleton compounds, as well as some intracellular alterations like, metabolic pathways, cell cytoskeleton rearrangement, organelle damage, vacuoles formation, activation of apoptosis and immunological pathways, DNA/RNA dysregulation or alterations, and as result the microorganisms could cause cell death. These responses by the host cell depend on the type of molecules presented by the pathogen (Figure 1).
For example, Uropathogenic Escherichia coli (UPEC) express several adhesins that allow the specific attachment on the urethra and bladder of the urinary tract. FimH is an adhesin from UPEC that is recognized by high-mannosylated glycoprotein UPIa on the uroepithelium cells, however, this adhesin can attach to a wide range of glycoproteins carrying at least one N-linked high-mannose structures [11,12]. FimH attach to its receptor facilitates the invasion, internalization, activation of apoptotic pathways, and induces cytoskeleton rearrangement in the uroepithelial cells (Klumpp et al., 2006)[13]. Likewise, the same fimbriae elicit production of some cytokines as TNF-α and IFN-γ, IL-6 and IL-8 for clearance and the inflammatory response in the bladder and in the kidney [14,15].
In addition, toxins and exoenzymes promote the infection and disease by direct or indirect damage to the host tissues [16]. These VF could be produced and released to the external media or anchored to cell membranes, viral envelope, or cell walls. The interaction of these molecules with the host could generate apoptosis, cell death, cytoskeleton disfunction, vacuole formation, activation of immune responses, DNA/RNA alteration, dysregulation, or damage in target organelles and finally, result in a loss of organ or systemic function, momentary or permanent, or even the death of the host. These molecules are essential for the development of the infection and disease in the cases of tetanus, aflatoxicosis, influenza, HIV, neosporosis, toxoplasmosis, etc. [16-19].
Conclusion
Hence, the cellular microbiology provides the techniques and strategies to identify pathogen virulence factors and their interaction with host. The fundamental principles generated from the understanding of host pathogen molecular cell microbiology research can lead us to the important discoveries in the novel vaccine development, the discovery of novel antimicrobial compounds, biomarkers, and diagnostic testing.
References
- Pizarro-Cerda J, Cossart P (2016) Cell biology and microbiology: a continuous cross-feeding. Trends Cell Biol 26(7): 469-471.
- Hogan L, Klein B, Levitz S (1996) Virulence factors of medically important fungi. Clinical Microbiology Reviews 9(4): 469-488.
- Staniszewska M (2020) Virulence factors in Candida species. Curr Protein & Pept Sci 21(3): 313-323.
- Klemm P, Schembri MA (2000) Bacterial adhesins: function and structure. Int J Med Microbiol 290(1): 27-35.
- Bouckaert J, Mackenzie J, de Paz J, Chipwaza B, Choudhury D, et al. (2006) The affinity of the FimH fimbrial adhesin is receptor-driven and quasi-independent of Escherichia coli Mol Microbiol 61(6): 1556-1568.
- Engelsoy U, Rangel I, Demirel I (2019) Impact of proinflammatory cytokines on the virulence of uropathogenic Escherichia coli. Front Microbiol 10: 1051.
- Mydock-McGrane LK, Cusumano ZT, Janetka JW (2016) Mannose-derived FimH antagonists: a promising anti-virulence therapeutic strategy for urinary tract infections and Crohn’s disease. Expert Opin Ther Pat 26(2): 175-197.
- Klumpp D, Rycyk M, Chen M, Thumbikat P, Sengupta S, et al. (2006) Uropathogenic Escherichia coli induces extrinsic and intrinsic cascades to initiate urothelial apoptosis. Infect Immun 74(9): 5106-5113.
- Dhakal B, Kulesus R, Mulvey M (2008) Mechanisms and consequences of bladder cell invasion by uropathogenic Escherichia coli. Eur J Clin Invest 38(Suppl 2): 2-11.
- Gupta RC (2018) Veterinary toxicology. In: Illustrated Toxicology. Academic Press, USA. pp. 427-517.
- Brown R, Priest E, Naglik J, Richardson J (2021) Fungal toxins and host immune responses. Front Microbiol 12: 643639.
- Ma L, Liu J, Li M, Fu Y, Zhang X, et al. (2017) Rhoptry protein 5 (ROP5) is a key virulence factor in Neospora caninum. Front Microbiol 8: 370.
- Beck B, Freudenreich O, Worth JL (2010) Patients with human immunodeficiency virus infection and acquired immunodeficiency syndrome. In: Massachusetts General Hospital Handbook of General Hospital Psychiatry. pp. 353-370.
- Cossart P, Boquet P, Normark S, Rappuoli R (1996) Cellular microbiology emerging. Science 271(5247): 315-316.
- Casadevall A, Pirofski L (2009) Virulence factors and their mechanisms of action: the view from a damage-response framework. J Water Health 7(Suppl 1): S2-S18.
- Bessaiah H, Pokharel P, Habouria H, Houle S, Dozois C (2019) yqhG Contributes to oxidative stress resistance and virulence of uropathogenic Escherichia coli and identification of other genes altering expression of type 1 Fimbriae. Front Cell Infect Microbiol 9: 312.
- Zilio G, Thievent K, Koella JC (2018) Host genotype and environment affect the trade-off between horizontal and vertical transmission of the parasite Edhazardia aedis. BMC Evol Biol 18(1): 59.
- Shapiro J, Williams E, Turner P (2016) Evolution of parasitism and mutualism between filamentous phage M13 and Escherichia coli. Peer J 4: e2060.
- Fink S, Campbell S (2018) Infection and host response. Molecular Pathology. (2nd edn), Academic Pres, USA, pp. 45-69.






























