Suppression of Estrogen Receptor in Breast Cancer Cells via Transferrin Coated Gold Nanoparticles Carrying Antisens Oligonucleotides
Ayse Kevser Piskin*
Faculty of Medicine, Lokman Hekim University, Turkey
Submission: February 24, 2022; Published: March 16, 2022
*Corresponding author: Ayse Kevser Piskin, Faculty of Medicine, Lokman Hekim University, Ankara, Turkey
How to cite this article: Ayse Kevser Piskin. Suppression of Estrogen Receptor in Breast Cancer Cells via Transferrin Coated Gold Nanoparticles Carrying Antisens Oligonucleotides. Curr Trends Biomedical Eng & Biosci. 2022; 20(3): 556040. DOI10.19080/CTBEB.2022.20.556040
Abstract
Breast cancer is the most common cancer of women. In breast cancer treatment the estrogen receptor is an established target in receptor positive tumors. A novel approach to therapy is gene regulation by oligonucleotide drugs. Antisens oligonucleotide therapy is facing with some difficulties such as targeting a polianionic nucleotide into the tumor cell. In order to form an efficient carrier system to protect and target antisens oligonucleotides against estrogen receptor α mRNA, a gold nanoparticle based construct is formed. Uptake of amino and thiol modified gold nanoparticles was analysed by confocal microscopy. Gold nanaparticles were functionalysed by transferrin coating and estrogen receptor α antisens oligonucleotide and were applied to MCF7 breast cancer cells. It was found that funtionalized gold nanoparticles decreased the estrogen receptor expression by 51% as well as cell proliferation upto 41.00 ± 5.6 % of untreated cells. Therefore, this targeting construct using the transferrin receptor and receptor mediated endocytosis process delivers the antisens oligonucleotide efficiently. Since estrogen receptor α is an important regulator of cell proliferation, this system is able to inhibit proliferation by means of decreasing the expression level of this major regulatory molecule. It is concluded that, this functionalysed gold nanoparticle system may be designed for other targets in tumors and may also be used as a teranostic agent.
Keywords: Gold nanoparticles; Breast cancer; MCF 7 cells; Estrogen receptor; Antisens oligonucleotides
Introduction
Breast cancer is the most common cancer of women and ranks as the second cancer related mortality after lung cancer [1-3]. Malignant transformation of normal breast cells requires disregulation of molecular and genetic processes. The wide distribution of these molecules including receptors, signal transducers and other proteins as well as genetic and epigenetic aberrations in different forms of breast cancer challenges clinicians. On the other hand, these molecular and genetic transformations provide new targets for breast cancer management [4]. Presently, there are numerous therapies for breast cancer treatment, including chemotherapy, immunotherapy, hormone therapy, surgery and radiotherapy. Among these therapies, hormone therapy which involves the application of drugs, for example tamoxifen and anastrozole, for inhibiting the production of estrogen or blocking the estrogen receptor is used to treat ER positive breast cancers [4,5]. Estrogen receptor (ERα) plays a critical role in mammary gland biology as well as breast cancer progression. It has been well documented that ERα has great potential to promote breast cancer cell motility and invasion and more than 70% of breast cancers are estrogen receptor positive [6].
During the past decades, nucleic acid-based technologies e.g. siRNA, antisens oligonucleotides (ASO, ASODN or ODNs), mRNAs, etc. have shown great potential for gene regulation [7-9]. A vast number of ODN delivery systems have been developed including viruses, metal and polymeric carriers [10,11]. The efficacy of carrier largely depends on not only its ability to carry the ODN by protecting its degradation and facilitating its uptake by the tumor but also targeting it specifically to the tumor. The concentrated work in ODN carrier research have expanded to searching for specific nucleic acid regulators and, teranostic carrier systems such as gold nanoparticles [12,13]. Gold nanoparticles (AuNPs) are of increasing interest due to their excellent biocompatibility and tunable optical chemistry and remarkable physicochemical properties. Because of these unique physical-chemical properties, the AuNPs are widely used as carriers of drugs and molecules to improve the diagnosis and treatment of diseases [14]. We aimed to design a gold nanoparticle carrier targeted to breast cancer cells to deliver antisens oligonucleotides which can suppress the estrogen receptor (ERα) expression. Cellular uptake of amino (NH2) and thiol (SH) modified AuNPs were analysed. The NH2 modified AuNPs were functionalized by binding transferrin and antisens ODN binding. These constructs are applied to estrogen receptor expressing MCF7 human breast cancer cells and the ERα expression and proliferation was determined.
Materials and Methods
Cells
MCF-7 human breast cancer cell line is obtained from ATCC (American Type Culture Collection). They were cultured in Dulbecco’s modified eagle medium (DMEM) -high glucose supplemented with 10% fetal bovine serum, 2 mM L-glutamine and 1% antibiotic-antimycotic solution in a 5% CO2 incubator at 37OC. Cells were passaged every two days and cell viability was monitored by trypan blue exclusion test and cell proliferation was measured by MTT test [15].
Preparation of AuNP constructs
AuNPs with SH and NH2 groups were purchased from Sigma Aldrich Co., 500 ng NH2 AuNPs were functionalized by binding 0.5 and 1 mg of Transferrin (TF) and 250 ng Antisens ODN (ASODN) having a sequence of 5'-GACCATGACCATGACCCT-3´by incubation at RT for 30 minutes in TE buffer at PH:7.0.
Confocal microscopy
MCF 7 cells were seeded at a density of 2x104 cells/ml on slides placed in 6 well tissue culture plates and were allowed to adhere for 24hr. Then, 100 ng ASODN, 100ng ASODN + AuNP –SH, 100ng ASODN + AuNP-SH + Transferrin, 100ng ASODN + AuNP -NH2, 100 ng oligo + AuNP-NH2 + Transferrin was applied to cells and cultured for another 24 hours. Then, the cells were fixed on the slide by applying 2.5% Glutaraldehyde solution for 5 minutes. Cells preparations in PBS were observed in PBS by confocal microscopy (Zeiss LSM 500) at 63X magnification.
Binding analysis
Binding of transferrin and ASODN to gold nanoparticles were analysed by gel retardation assay free and auNP bound transferrin were run in 10% SDS PAGE and the bands were stained with coomassie blue [16]. Estrogen ASODN binding to AuNPs with and without transferrin was also analysed by retardation in 10% polyacrylamide gel and detected by silver staining [17]. Briefly, gel staining was completed by fixing in a solution containing 40% ethanol, 10% acetic acid asetik as it in distilled water and sensitized in 0.02% sodium thiosulphate for 60 minutes and stained with silver nitrate for 20 minutes. Gel was visualized by with developing solution containing 3% sodium carbonate and 0.05 % formaldehyde.
Expression analysis
Expression of ER-α was analysed by real time PCR using Rotor Gene Q apparatus (Qiagen, Netherlands). Total cellular RNA was isolated from MCF-7 cells by using RNA easy Mini Kit (Qiagen, Netherlands). RNA yield was determined by spectroscopy. Complementary DNA was made from 2,5µg of total RNA using RT2 First Strand Kit. (Qiagen, Netherlands) Real-Time PCR (RT-PCR) was performed using SYBR Green PCR Master Mix and message level was determined using the DDCt method. RT-PCR was carried out under the following reaction conditions for ER-α and GAPDH primers, stage 1, 95OC for 10 min (Rep 1); stage 2, 95 OC for 15 s then 60 OC for 1 min (Reps40).
Primer sequences used in the experiment were as follows;
ER- α: Forward 5'- TGGGCTTACTGACCAACCTG -3'
Reverse 5'-CCTGAT CATGGAGGGTCAAA -3'
GAPDH: Forward 5'- CGGAGTCAACGGATTTGGTCGTAT-3'
Reverse 5'- AGCCTTCTCCAT GGTGGTGAAGAC-3'
Data normalization was performed by using the threshold Cycle value (Ct) of human GAPDH (Ct - Ct GAPDH = DCt). DCt values for each sample were then normalized to control samples. Results were expressed as an n-fold change in gene expression relative to control samples [18]. Primers and all reactive were obtained as a kit (RT2 SYBR Green ROX FAST Master mix from Qiagen, Netherlands).
Data analysis
Statistical evaluation of results were made by using SPSS 15 software and p<0.05 is considered as significant. RT-PCR data were analysed by Friedman test and cell proliferation was determined by variation analyses and compared by Bonferroni test.
Results and Discussion
Binding of transferrin and antisens oligonucleotides to gold nanoparticles
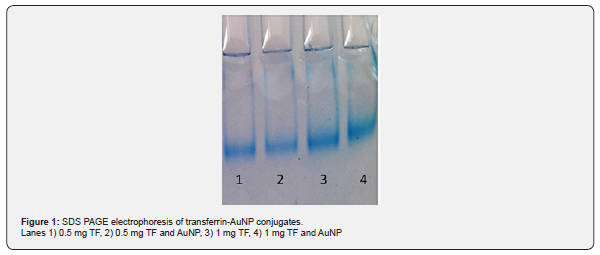
Transferrin-conjugated nanoparticles are bioconjugates as a vehicle for specific cellular uptake and targeted drug delivery since most cancer cells have high expression of transferrin receptors. Transferrin bound AuNPs moved slower in gel retardation test as seen in Figure 1. Binding of transferrin to AuNps was concentration dependent as depicted from the figure. Binding of estrogen receptor α ASODN to gold nanoparticles with or without transferrin was also observed in gel retardation assay as seen in Figure 2. The major challenge in ASODN delivery to cancer cells is protecting them from nuclease attack. Moreover, the hydrophilic character and anionic backbone of ASODNs is a drawback for cellular uptake. Gold nanoparticles carrying amino groups on their surface may enhance cellular uptake and transferrin may play a dual role by binding and protecting ASOSs on the gold surface and targeting them into the tumor cell via receptor mediated endocytosis.

Cellular uptake of gold nanoparticle constructs
Estrogen α receptor ASODNs were bound to gold nanoparticles which were modified by amino or thiol groups and transferrin. Their cellular uptake was analysed by confocal microscopy. It is found that gold nanoparticles were readily taken by cells (Figure 3). Efficiency of transferrin targeting maybe shown more clearly by in vivo studies. Targeting by transferrin is considered as a suitable approach as transferrin coated nanoparticles that carry drugs were efficiently transferred to different kinds of tumors [19,20]. Especially gold nanoparticles may also be used in the diagnosis of tumors. The teranostic potential of gold nanoparticles have been demonstrated for a variety of tumors [21,22]. Therefore, transferrin coated AuNPs may provide efficient tools for specific monitoring and treating cancer cells.
Estrogen α receptor expression
Estrogen receptor mRNA was determined by RT-PCR (Figure 4). It is found that 24 hr treatment with only ASODN, AuNP bound ASODN and transferrin coated AuNP bound ASODN has led to a decrease of 15%, 37% and 51% in estrogen receptor α expression. These differences in expression were found to be significant (p<0.05; n=4).
Cell proliferation
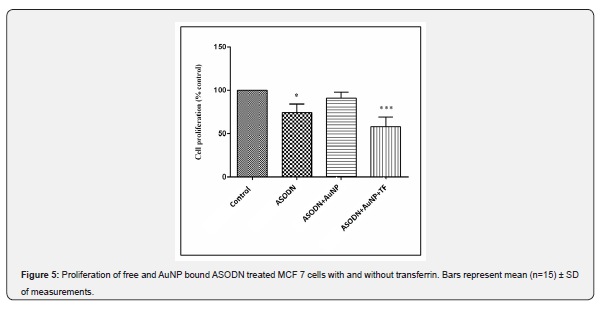
Application of ASODNs and ASODN bound transferrin coated AuNPs resulted in a decrease in MCF 7 cell proliferation (Figure 5). As shown in the figure 48 hours of treatment with ASODN, AuNP bound and transferrin coated AuNP bound ASODN have decreased cell proliferation by 22.00±6.9, 8.00±3.55 and 41±5.6 percent of control respectively. Difference between the effect of free and in transferrin coated AuNP construct form ASODN were found to be significant (p<0.05). Antisense against ERα was shown to decrease the proliferative ability of MCF-7 cells, since their proliferation is controlled by ERα-mediated gene regulatory pathways as shown previously [23]. Here, the AuNp construct with transferrin and ASODN is shown to be most effective antiproliferative agent. Gene therapy is one of the most exiting advances in medical biotechnology. Since 1998 more than 20 different kinds of gene therapy drugs are developed. Most of them are based on viral vectors. In the past few years, the approved gene therapy drugs have been effective in clinical applications. However, the costly and cumbersome process in manufacturing process of nucleic acid drugs including other RNA based drugs like siRNA remains to be a major problem (Kassner et al. 2018; Senior 2017). Novel efficient ODN drug delivery systems may contribute greatly to making these drugs more available for patients as these new gene therapy products based on specific vectors may offer highly efficient biotechnology at a lower cost [24,25].


References
- Jemal A, Siegel R, Ward E, Murray T, Xu J, et al. (2006) Cancer Statistics. CA Cancer J Clin 56 (2): 106-130.
- Hanahan D, Weinberg RA (2000) Hallmarks of Cancer. Cell 100(1): 57-70.
- Siegel RL, Miller KD, Jemal A (2020) Cancer Statistics. CA Cancer J Clin 70(1): 7-30.
- Castro NP, Osorio CA, Torres C, Bastos EP, Neto MM, et al. (2008) Evidence that molecular changes in cells occur before morphological alterations during the progression of breast ductal carcinoma. Breast Cancer Res 10(5): R87.
- De Santis C, Siegel R, Bandi P, Jemal A (2011) Breast Cancer Statistics. CA Cancer J Clin 61(6): 409-418.
- Liao XH, Lu DL, Wang N, Liu LY, Wang Y, et al. (2014) Estrogen receptor a mediates proliferation of breast cancer MCF–7 cells via a p21/PCNA/E2F1-dependent pathway. FEBS J 281(3): 927-942.
- Liu T, Song S, Wang X, Hao J (2021) Small-molecule inhibitors of breast cancer-related targets: Potential therapeutic agents for breast cancer. Eur J Med Chem 210: 112954.
- Kim J, Hu C, El Achkar CM, Black LE, Douville J, et al. (2019) Patient-customized oligonucleotide therapy for a rare genetic disease. N Engl J Med 381(17): 1644-1652.
- Le TK, Paris C, Khan KS, Robson F, Ng WL, et al. (2021) Nucleic acid-based technologies targeting coronaviruses. Trends Biochem Sci 46(5): 351-365.
- Roberts TC, Langer R, Wood MJ (2020) Advances in oligonucleotide drug delivery. Nat Rev Drug Discov 19(10): 673-694.
- Dincer S, Oskay EK, Piskin AK, Zeybek ND, Piskin E (2010) Growth inhibition of SK-MEL-30 human melanoma cells by antisense c-myc oligonucleotides delivered by poly(N-isopropylacrylamide)/ poly(ethyleneimine) copolymer. J Tissue Eng Regen Med 4(4): 284-290.
- Madamsetty VS, Mukherjee A, Mukherjee S (2019) Recent trends of the bio-inspired nanoparticles in cancer theranostics. Front Pharmacol 10: 1264.
- Norouzi M (2020) Gold nanoparticles in glioma theranostics. Pharmacol Res 156: 104753.
- Chen W, Zhang S, Yu Y, Zhang H, He Q (2016) Structural-engineering rationales of gold nanoparticles for cancer theranostics. Adv Materials 28(39): 8567-8585.
- Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65(1-2): 55-63.
- Botteon CE, Silva LB, Ccana-Ccapatinta GV, Silva TS, Ambrosio SR, et al. (2021) Biosynthesis and characterization of gold nanoparticles using Brazilian red propolis and evaluation of its antimicrobial and anticancer activities. Nat Scienti Rep 11: 1974.
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nat 227(5259): 680-685.
- Chevalet M, Lucke S, Rabillaud J (2006) Silver staining of proteins in polyacrylamide gels. Nat Protoc 1(4): 1852-1856.
- Nolan T, Hands E, Bustin SA (2006) Quantification of mRNA using real-time RT-PCR. Nat Protoc 1(3): 1559-1582.
- Bhagwat GS, Athawale RB, Gude RP, Shadab M, Nabil AA, et al. (2020) Formulation and development of transferrin targeted solid lipid nanoparticles for breast cancer therapy. Front Pharmacol 11: 614290.
- Dixit S, Novak T, Miller K, Zhu Y, Kenneyc ME, et al. (2015) Transferrin receptor-targeted theranostic gold nanoparticles for photosensitizer delivery in brain tumors. Nanoscale 7(5): 1782-1790.
- Akhter S, Ahmad MZ, Ahmad FJ, Storm G, Robbert J (2012) Gold nanoparticles in theranostic oncology: current state-of-the-art. Expert Opin Drug Deliv 9(10): 1225-1243.
- Unak G, Ozkaya F, Ilker ME, Kozgus O, Sakarya S, et al. (2012) Gold nanoparticle probes: design and in vitro applications in cancer cell culture. Colloids Surf B Biointerfaces 90: 217-226.
- Carroll JS, Swarbrick A, Musgrove EA, Sutherland RL (2002) Mechanisms of growth arrest by c-myc antisense oligonucleotides in MCF-7 breast cancer cells implications for the antiproliferative effects of antiestrogens. Cancer Res 62(11): 3126-3131.
- Maa CC, Wang ZL, Xua T, He ZY, Wei YQ (2020) The approved gene therapy drugs worldwide: from 1998 to 2019. Biotechnol Adv 40: 107502.






























