Optimal Inventory Strategies for Pharmaceutical Products Incorporating Carbon Emissions
Adekunle Odunayo Adejuwon1* and Victoria Anatolyivna Tsygankova2
1School of Health Information Management, University College Hospital
2Department for Chemistry of Bioactive Nitrogen-Containing Heterocyclic Compounds, Institute of Bioorganic Chemistry and Petrochemistry of the National Academy of Sciences of Ukraine, Ukraine
Submission: April 02, 2019; Published: June 11, 2019
*Corresponding author: Adekunle Odunayo Adejuwon, School of Health Information Management, University College Hospital, Ibadan, Nigeria
How to cite this article: Adekunle O A, Victoria A T. α-Amylase Production Using Aspergilus vadensis Isolated from Pulverized Cocoa Seeds. Curr Trends 002 Biomedical Eng & Biosci. 2019; 19(2): 556010. DOI: 10.19080/CTBEB.2019.19.556010
Abstract
Aim: α-Amylases are well known for their applications ranging from food and paper industries to pharmaceutical industries.
Materials and Methods: Aspergillus vadensis isolated from pulverised cocoa seed was employed in the submerged fermentation of plantain peel. The pH and temperature of the extract were measured at two days (48 hrs) interval using pH meter and sugar concentration was also determined using Fehling test. Protein content and α-amylase activity of the crude extract was determined.
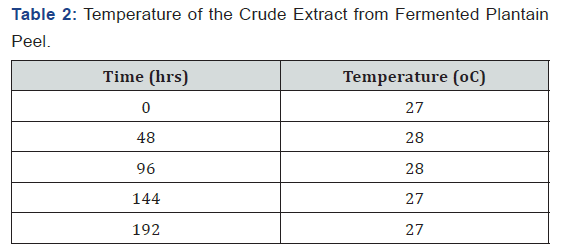
Results:The pH of the crude extract decreased from 4.78 on the first day to 2.51 on day eight (192 hrs) while the temperature of the extract fluctuated between 27 oC and 28 oC. The sugar concentration in the extract increased from 3.36 mg/ml after 48 hrs to 26 mg/ml after 144 hrs and declined to 8.02 mg/ml after 192 hrs. The α-amylase activity also reached its peak (68.86 U/mg protein) on the 6th day (144 hrs) but declined to 50 U/mg protein on the 8th day (192 hrs).
Acknowledgements: It can be concluded that plantain peel can be employed as a cheap and readily available substrate in the production of α-amylase that is used for various biotechnologically-based industrial applications thereby, adding value to plantain and also decreasing the amount of this agro industrial waste in the environment.
Keywords: Plantain peel; α-amylase; Aspergillus vadensis
Introduction
α-Amylases are enzymes that are well known for their applications in starch and food, brewing, distilling, textile, paper and pharmaceutical industries [1-3]. This large range of applications is the triggering factor for the industrial production of this enzyme [4]. Presently the enzyme is one of the mostly sought after as it has great significance in biotechnology; constituting a class of industrial enzymes that controls about 25% of the world’s total enzyme market [5,6]. Agro industrial wastes have been reported to be good substrates for the cost effective production of alpha amylases [2] and are thus attracting researchers for using agro industrial waste as a substrate for alpha amylase production. Agricultural wastes include but are not limited to plantain peel, wheat bran, rice husk, banana peel, vegetable waste and citrus waste. A wide range of microbes, such as bacteria and fungi are used for the industrial production of amylases [1]. The use of microorganisms for the production of amylases is economical as microbes are easy to manipulate to obtain enzymes of desired characteristics [7]. However, fungi are preferred over bacteria for enzyme production because of their filamentous nature, which helps in their penetration through solid substrate [8].
Plantain (Musa spp.) occupies a strategic position for rapid food production in Nigeria. It is ranked third among starchy staples [9]. Total world production of plantain is estimated to be over 75 million metric tons [10]. Twelve million metric tons are produced in Africa annually [11]. Nigeria is one of the largest plantain producing countries in the world. It is the largest producer in West Africa with annual production of about 2.4 million metric tons mostly obtained from the Southern States [12]. However, Nigeria does not feature among plantain exporting nations because it produces more for local consumption than for export [13]. Plantain is a major source of carbohydrate for more than 50million people [14]. Besides being the staple for many people in more humid regions, plantain is a delicacy and favoured snack for people even in other ecologies [9]. In Nigeria, the ripe fruit are processed into different forms for consumption either by boiling, frying or roasting. The peel of the fruit is discarded as waste after the inner fleshy portion has been eaten, thereby constituting a menace to the environment, especially where its consumption is common [15]. According to FAO [16] about 17,397,000 metric tonnes of plantain peels are generated in African countries.
The accumulation of the discarded peel may lead to the generation of domestic waste which could constitute health hazards in different parts of the country. Hence, this study aims at generating additional value to plantain by utilizing the peel as a substrate for the production of the enzyme amylase in a view to converting this agricultural and potentially hazardous waste into wealth.
Materials and Methods
Isolation of Aspergillus vadensis from cocoa
The isolate of Aspergillus vadensis used for this investigation was isolated from pulverised cocoa seeds. Potato dextrose agar (PDA) media was prepared, autoclaved and poured in sterile Petri dishes. One gram of the pulverised cocoa seeds was transferred into 20 mL of sterile distilled water. 0.1 mL from the mixture was transferred into the prepared PDA and the plates were incubated at 27 oC for 72 hours. Nine different isolates were observed on the incubated plates, Aspergillus vadensis used for this study was identified based on its physical and microscopic (Lactophenol cotton blue) characteristics [17]. This was used as a form of preliminary identification. Aspergillus vadensis was further sub-cultured on a freshly prepared PDA and incubated at 27 oC for 72 hours [18].
Processing of plantain peel substrate
The ripe plantain purchased from the local market was peeled off and the pulp (fruit) was separated. The peels were thoroughly washed using sterile distilled water and were cut into small pieces followed by homogenization in a blender. Water was added to facilitate the homogenization.
Sub merged fermentation
Fermentation was carried out using the modified method of Khan & Yadav [4]. Thirty gram (30 g) each of the paste of plantain peel was dispensed into eight different 250 mL conical flasks and about 70 mL of basal medium containing the following in g/l (0.8 g NaCl, 0.8 g KCl , 0.1 g CaCl2, 2.0 g Na2HPO4, 0.2g MgSO4, 0.1 g FeSO4, 8.0g Fructose, 2.0 g NH4Cl was added. The contents in conical flasks were autoclaved, cooled at room temperature, inoculated with 1 mL of 72 hours old grown culture and incubated at 27 oC. The contents of one of the conical was not inoculated with Aspergillus vadensis, this was used as the control for comparison.
Determination of physical parameters
pH and temperature: The pH of the crude extract was determined using pH meter PHS -3E (Surgifriend Medicals, England) at 2 days (48 hrs) interval.
'Determination of sugar concentration
Different concentrations of glucose used as standard were prepared from 5g of D-glucose dissolved in 45ml of distilled water. The sugar contents of the D-glucose and crude extract were determined, using Fehling’s sugar test method. 20ml of prepared Fehling solution “A” was mixed with 20 ml of Fehling solution “B”. 2ml of the Fehling solution mixture was added to 5 test tubes containing the different concentrations of D glucose used as standard, and the diluted crude. The test tubes were placed in a water bath at 60 °C for 15min after which absorbance was read by spectrophotometer at a wavelength of 500 nm.
Extraction of crude enzyme
Extraction of crude enzyme was done according to the modified method of Adejuwon [19]. Contents of the flask was filtered using Whatman no. 1 filter paper in a cold chamber at 4 oC. The extract was then subjected to cold centrifugation at 10,000 rpm for 10 minutes at 4 oC using a high speed cold centrifuge. This served as the crude enzyme. Amylase activity was determined [20]. The protein content of the preparation was determined [21].
Protein estimation in crude enzyme
Concentration of protein in crude enzyme was determined by the method of Bradford [21] in which crude enzyme was reacted with Bradford reagent and the absorbance obtained was compared with a standard graph plotted by reacting a standard protein with known concentrations with the Bradford reagent and plotting a graph between concentration of standard protein (Bovine Serum Albumin) on X axis and absorbance at 595 nm on Y axis
α-Amylase assay in crude enzyme
α-Amylase activity was assayed using the modified method of Pfueller & Elliott [20] and Adejuwon et al. [22]. The reaction mixture was 2 mL of buffered (0.02m citrate phosphate buffer pH 6.0), soluble starch (Sigma) and 0.5 mL enzyme. The control consisted of only the prepared substrate. Incubation was at 35 °C for 30 minutes. The reaction was terminated with 3 mL of 1 N HCl. 2 mL of the terminated reaction mixture was added to 3 mL of 0.1N HCl. Colour was developed by adding 0.1ml iodine solution. Controls consisted of only 2 mL of the prepared substrate. One unit of α-amylase activity was defined as the amount of enzyme which produced 0.01% reduction in the intensity of the blue colour of the starch-iodine complex under assay conditions.
Results
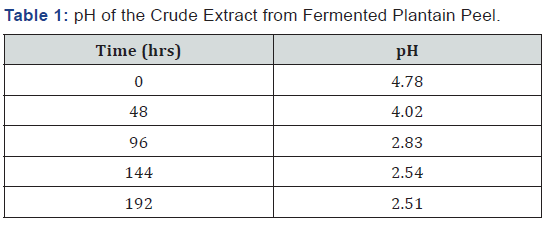
The pH of the crude extract from the fermenting media was measured using a pH meter after every two days (48 hrs) and as shown in Table 1, the pH decreased as fermentation progresses. The temperature of the crude extract from the fermenting media was measured using a thermometer after every two days (48 hrs) and as shown in Table 2, the temperature fluctuated between 27 oC and 28 oC as fermentation progressed. The sugar concentration in the crude extract from the fermenting media was determined using a Fehling test after every two days and as shown in Table 3, the sugar concentration reached its peak on the sixth day after fermentation and declined on the eight day. The crude extract from the fermenting media was assayed for α-amylase activity using the method of Pfueller & Elliott [20]. This was done after every two days (48 hrs) and as shown in Table 4, α-amylase activity reached its peak on the sixth day (144 hrs) after fermentation and declined on the eight day (192 hrs).
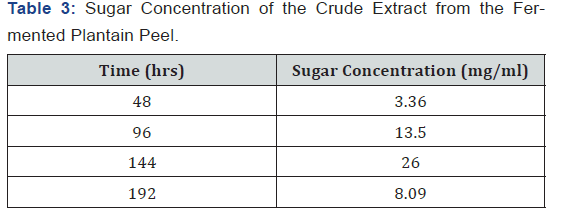
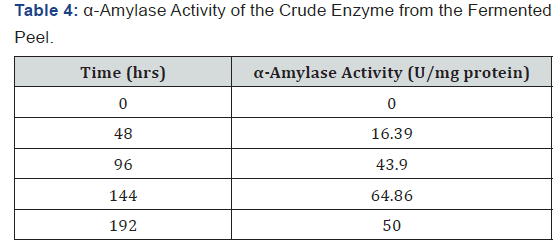
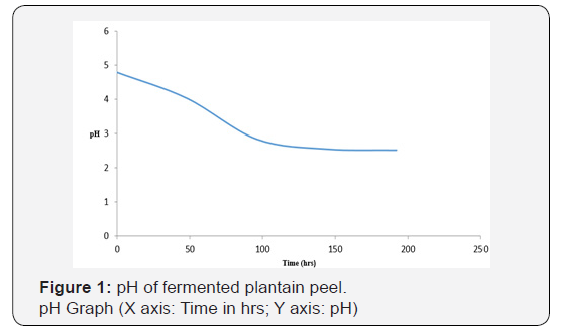
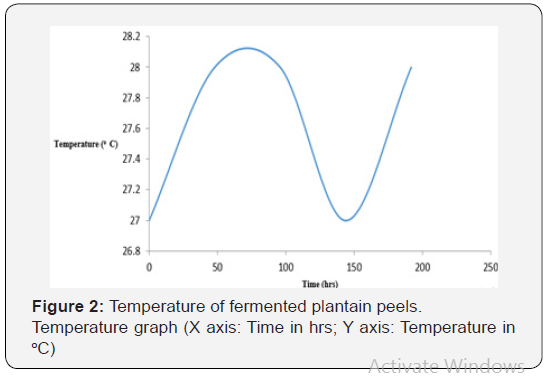
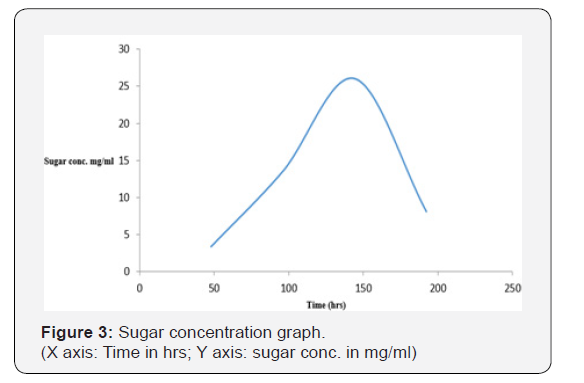
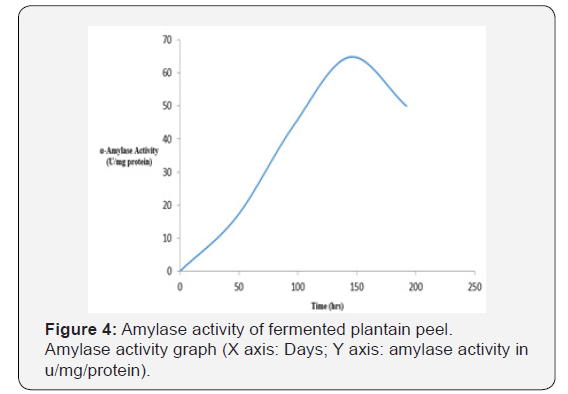
As shown in Figure 1, the initial pH decreased from 4.78 on the first day to 2.51 on day eighth (192 hrs). This may be due to production of acid and alcohol as a result of prolonged fermentation. As shown in Figure 2, the temperature in the crude extract varied between 27 oC and 28 oC. As shown in Figure 3, the sugar concentration in the extract from the fermented peel increased from 3.6 mg/ml after 48 hrs to 26 mg/ml after 144 hrs. It declined to 8.09 mg/ml on the 8th day. As shown in Figure 4, the α-amylase activity of the crude from the fermented peel increased from 0 U/mg protein on the first day to 64 U/mg protein on the sixth day. It declined to 50 U/ mg protein on the eighth day.
Discussion
Plantain peels are by-products of the plantain-processing industry, which are normally dumped in landfills, rivers or un regulated grounds [23]. The bio-accumulation of this waste may pose serious environmental problem; hence, there is a need for effective utilization of this waste. Agro industrial wastes such as banana peel and cassava peel have been reported to be good substrate for amylase production [4,24]. The expression of α-amylase activity by Aspergillus vadensis with plantain peel as the sole substrate is an indication of constitutive expression or induction of the enzyme in the fungus. α-Amylase production by microorganisms may be constitutive or inductive [25,26]. The pH in the fermenting sample declined as the fermentation progressed; this could be as a result of acid and alcohol production due to prolonged fermentation.
The sugar concentration attained its peak 26 mg/ml on day six after fermentation (144 hrs) and declined to 8.09 mg/ml on the eight day, this showed that the fungus is able to optimally utilize the sugar present in the fermenting sample on the 6th day due to the complete hydrolysis of the substrate, this reflected in the α-amylase activity which also reached its peak 64.86 U/mg protein on the 6th day.
The decline in α-amylase activity from 64.86 U/mg protein on the 6th day to 50 U/mg protein on the 8th day (192 hrs) could be attributed to the decline in the sugar concentration in the fermenting sample. It could also be attributed to depletion of nutrient and also accumulation of by-products such as toxins and inhibitors [27,28].
Elmarzugi et al. [29] reported that amylase contributed about 25-30% to a US$ 2.7 billion world enzyme market as at 2012 which is estimated to increase by 4% annually. Considering the fact that Nigeria is one of the largest producers of plantain in Africa [12], this study is important as it will not only add value to plantain and help reduce environmental problems but could also be a source of revenue for Nigeria as it looks to diversity in its economy.
Conclusion
It can be concluded that plantain peel can be employed as a cheap and readily available substrate in the production of α-amylase that is used for various industrial applications thereby, adding value to plantain and also decreasing the amount of this agro industrial waste in the environment. Future prospects of the present study include optimization of pH and optimization of the sugar concentration in order to maintain a constant production for a longer period
Acknowledgements
Authors are grateful to the Institute of Bioorganic Chemistry and Petrochemistry of the National Academy of Sciences (NAS) of Ukraine, East Europe for Research Supports.
References
- Krishna PR, Srivastava AK, Ramaswamy NK, Suprasanna P, Souza SFD (2011) Banana peel as substrate for alpa-amylase production using Aspergillus niger NCIM 616 and process optimization. Indian Journal of Biotechnology 11: 314-319.
- Pandey A, Nigam P, Soccol CR, Soccol VT, Singh D, et al. (2000) Advances in microbial amylases. Biotechnol. Appl Biochem 31(2): 135-152.
- Gupta R, Gigras P, Mohapatra H, Goswami VK, Chauhan B (2003) Microbial - amylases: a biotechnological perspective. Process Biochem 38(11): 1599-1616.
- Khan JA, Yadav SK (2011) Production of alpha amylases by Aspergillus niger using cheaper substrates employing solid state fermentation. International Journal of Plant, Animal and Environmental Sciences 1(3): 100-108.
- Reddy NS, Nigmmagadda A, Sambasiva Rio KRS (2003) An overview of the microbial α–amylase family. African J Biotechnol 2: 645-648.
- Rajagopalam G, Krishnan C (2008) Alpha-amylase production from catabolite depressed B. subtilis Kcc 103 utilizing sugar-cane bagasse hydrolysate. Bioresource Technol 99(8): 3044-3050.
- Aiyer PV (2005) Amylases and their applications. Afr J Biotechnol 4: 1525-1529.
- Ramachandran S, Patel AK, Nampoothiri KM, Francis F, Nagy V (2004) A potential raw material for the production of alpha-amylase. Bioresource Technol 93: 169-174.
- IITA: International Institute of Tropical Agriculture (2014) A Reference Manual on Plantain Cultivation in West Africa 1-28.
- John P, Marchal J (1995) Ripening and biochemistry of the fruit, In: Gowen SR (Eds.), Bananas and Plantains, Chapman and Hall, London, U.K.
- Fakayode BS, Rahji MAY, Ayinde O, Nnom GO (2011) An economic assessment of plantain production in Rivers State, Nigeria. Int J Agric Econ Rural Dev 4(2).
- FAO: Food and Agriculture Organization of the United Nations (2006) Production Yearbook 2004. FAO, Rome.
- Fortaleza C (2012) The ultimate wealth guide to making millions of Naira yearly with plantain farming in Nigeria.
- Adeolu AT, Enesi DO (2013) Assessment of proximate, mineral, vitamin and phytochemical compositions of plantain (Musa paradisiaca) bract - an agricultural waste. International Research Journal of Plant Science 4(7): 192-197.
- Okareh OT, Adeolu AT, Adepoju OT (2015) Proximate and mineral composition of plantain (Musa paradisiaca) wastes flour; a potential nutrients source in the formulation of animal feeds. African Journal of Food Science and Technology 6(2): 53-57.
- FAO: Food and Agriculture Organization of the United Nations (1988) Production Yearbook 42, FAO, Rome.
- Cannon PF, Kirk PM (2007) Fungal Families of the World. CAB International Publishing, Wallingford, Oxfordshire, Pp 45-56.
- Adejuwon AO, Oluduro AO, Agboola FK, Ajayi AA, Olutiola PO, et al. (2015) Expression of alpha-amylase by a tropical strain of Aspergillus niger: Effect of carbon source of growth. Nat Sci 13(8): 66-69.
- Adejuwon AO (2010) Synthetic production of amylase from Aspergillus niger isolated from citrus fruit. African Journal of Basic and Applied Sciences 2(5-6): 158-160.
- Pfueller SL, Elliott WH (1969) The extracellular amylase of Bacillus stearothermophilus. J Biol Chem 224(1): 48-54.
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248-254.
- Adejuwon AO, Tsygankova VA, Alonge O (2018) Effect of cultivation conditions on activity of α-amylase from a tropical strain Aspergillus flavus Link. Journal of Microbiology, Biotechnology & Food Sciences 7(6): 571-575.
- Osma JF, Herrera JLT, Couto Sr (2007) Banana skin: A novel waste forlactase production by Trametspubescens under solid-state conditions. Application to synthetic dye decolouration. Dye & Pigments 5: 32-37.
- Brisibe EA, Bankgong H (2014) Biotechnological potential of alpha amylase production by Bacillus subtilis using cassava peel powder as a substrate. British Biotechnology Journal 4(11): 1201-1211.
- Dixon M, Webb EC (1971) Enzymes. Longmans, London, Pp 950.
- Dunn G (1974) A model for starch breakdown in higher plants. Phytochem 13: 1341-1346.
- Mulimani VH, Patil G, Ramalingan N (2000) Alpha amylase production by solid state fermentation: A new practical approach to biotechnology sources. Biochem Adv 28: 161-163.
- Teodoro CE, Martins LL (2000) Culture conditions for the production of thermostable amylase by Bacillus species. Braz J Microbiol 31: 298-2302.
- Elmarzugi NA, El Enshasy HA, Abdul Hamid M, Hasham R, Aziz A, et al. (2014) A amylase economic and application value. World Journal of Pharmaceutical Research, 3(3): 4890-4906.






























