Is Laccase Enzyme an Answer for Sustainable Thatch Management in Turfgrass Systems: A Review
Sudeep Singh Sidhu1,2*
1Department of Crop and Soil Sciences, The University of Georgia, USA
2North Florida Research and Education Center, University of Florida, USA
Submission: March 01, 2019; Published: April 09, 2019
*Corresponding author: Sudeep Singh Sidhu, University of Florida, North Florida Research and Education Center, Quincy, FL 32351, USA
How to cite this article: Sudeep S S. Is Laccase Enzyme an Answer for Sustainable Thatch Management in Turfgrass Systems: A Review. Curr Trends Biomedical Eng & Biosci. 2019; 19(1): 556002. DOI: 10.19080/CTBEB.2019.19.556002
Abstract
Use of laccase enzyme as a potential technique to manage thatch layer in turfgrass systems has been investigated in recent years. Thatch layer is a layer of organic matter formed between the soil and the green turfgrass. Presence of lignin, a recalcitrant component of organic matter, acts as the rate limiting step in organic matter degradation. Detrimental effects of a thick layer of thatch has been well documented which merits its effective management. Several costly, labor intensive, and destructive cultural practices are in use by industry to effectively control thatch. The destructive nature of these cultural practices presented an opportunity to investigate non-destructive and ecofriendly methods to manage thatch. This article reviewed unique characteristics of laccase enzyme, its ability to effectively degrade lignin, and development of laccase as a potential technique to effectively manage thatch layer in turfgrass systems.
Keywords: Laccase; Lignin; Thatch layer; Dethatch; Organic matter; Creeping Bentgrass; Agrostis stolonifera L.; Bermudagrass; Cynodon dactylon L.; Zoysiagrass; Zoysia japonica Stued
Introduction
Lignin, a major component of plant cell wall, acts as a protective matrix and limits microbial degradation of cellulose and hemi-celluloses which are readily degraded by microbes [1]. Heterogenous and complex structure of lignin macro-molecule which is due to random coupling of three monomers makes it resistant to microbial degradation and hence a rate limiting step in organic matter decomposition [2-5]. Formation of thatch layer in turfgrass systems is accelerated when organic matter accumulation rate exceeds its degradation rate. Excessive thatch layer accumulation leads to physical conditions in thatch which are detrimental to turfgrass [6]. Cultural management practices such as core aeration, vertical mowing, and grooming are destructive in nature and adversely impact turf quality.
Several non-destructive studies in the past utilized glucose, cellulase, mixture of amino acids, and microbial inocula to enhance organic matter decomposition in thatch layer. These studies proved futile as they were targeting decomposition of cellulose and hemi-cellulosic components instead of lignin [1,7]. Certain white-rot fungi produce extra-cellular enzymes known as lignolytic enzymes which are capable of lignin degradation [8]. Mechanism of most of the lignolytic enzymes such as lignin peroxidases (LiP; EC1.11.1.14), manganese peroxidases (MnP; EC 1.11.1.13), and versatile peroxidases (VP; EC 1.11.1.16) is hydrogen peroxide dependent [9]. The requirement of using hydrogen peroxide, a strong oxidant, to carry out the reaction of these enzymes makes them unusable in turfgrass systems due to phytotoxicity. Laccases (Lac; EC 1.10.3.2) have a unique mechanism and presented the opportunity to be utilized in turfgrass systems as a direct surface application [10]. This article reviewed complex structure of lignin, structure and unique oxidation mechanism of laccase enzyme that makes it suitable to be used in turfgrass systems, and developmental progress of laccase as a technique to manage thatch layer.
Lignin and Laccase
Lignin is the second most abundant organic substance next to cellulose and the major contributor to lignocellulosic recalcitrance to microbial degradation. Lignin is a three-dimensional amorphous polymer which is composed of three lignin monomers: p-caumaroyl, coniferyl, and sinapyl alcohols. The corresponding lignin monomers are known as p-hydroxy phenyl, guaiacyl and syringyl units, respectively, and often abbreviated as H, G, and S lignin [11]. The ratio of G:S:H is generally 70:25:5 in the lignocellulosic materials [12]. The recalcitrant nature of lignin is attributed to its heterogeneous complex structure, which is derived from random oxidative coupling of lignin monomers and cross-linking of polymers. A lignin macromolecule contains monolignols randomly bonded by C-O-C and C-C linkages including β-O-4, β-5, β-β, 5-5, 4-O-5, and β-1 bonds [2,3].
Fungal laccases occur as monomeric or dimeric protein structures with four copper atoms per molecule. The monomeric protein structures have a molecular mass of 50 to 100 kDa [13].
The process of laccase catalysis occurs in presence of oxygen in three steps:
1) Reduction of type I Cu by substrates;
2) Electron transfer from type I Cu to type II and III Cu trinuclear cluster; and
3) Reduction of oxygen to water at the trinuclear cluster [14].
Efficacy of laccase enzyme is dependent on its redox potential. Laccase produced from fungal sources have higher redox potential and is used in several biotechnological and environmental studies [13,15,16]. Redox potential of laccase enzyme from different fungi is reported from 450 mV to 800 mV [9]. Low redox potential of fungal laccase restricts its ability to oxidize non-phenolic compounds [17]. However, addition of low molecular weight substances, known as mediators, increase the substrate range of laccase enzyme to non-phenolic groups, benzyl and alyl alcohols and ethers [18-20] which contribute the major fraction of the lignin macromolecule [11,12]. As laccase enzyme requires oxygen instead of a strong oxidant as part of its mechanism to oxidize organic molecules, this property makes laccase unique to be utilized in agricultural systems.
A novel non-destructive approach was developed at The University of Georgia, Griffin Campus to utilize laccase enzyme alone or along with mediators as a direct application on several turfgrasses maintained as home lawns or recreational turf [21,22]. For all the studies, laccase from fungus Trametes versicolor was used due to its high redox potential (800 mV). The results of these studies will be discussed in the following sections.
Proof of Concept [23]

A greenhouse study was conducted on ‘Crenshaw’ creeping bentgrass (Agrostis stolonifera L.) pots. Treatment application included direct spay of laccase enzyme with or without a mediator, guaiacol. Treatments were applied once every two weeks which included 40-mL solution of laccase at activity levels 0, 0.206, and 2.06 units cm-2 and 10-mL solution of guaiacol at 0 and 0.1 M concentration. Samples were analyzed after two and nine months of treatment application to observe the impact of laccase enzyme on thatch layer and turfgrass quality. No significant differences were observed for any of the treatments after two months of application. After nine months of application, laccase treatment at lower activity level of 0.206 units cm-2 was not effective. However, laccase treatment at activity level of 2.06 units-2 reduced thatch layer thickness by 45% when compared to control pots. Treatment with 2.06 units cm-2 laccase with and without guaiacol reduced organic matter content in the top 2.5 cm thatch layer by 25.9 and 30.3 mg·g-1, respectively compared to control (Figure 1). Laccase treatment also reduced lignin content by 19.0 mg·g-1 compared to control. No adverse effect was observed on turfgrass quality for the duration of the experiment. Application of mediator guaiacol had slight impact on overall efficacy of laccase enzyme when samples were analyzed after nine months. This study provided answers in terms of laccase activity and application duration needed to impact turfgrass thatch layer and if there were any adverse phytotoxicity effects of direct application of laccase.
Thatch composition altered by laccase [24]
This study was conducted on dead creeping bentgrass pots to observe the impacts of laccase application on thatch layer composition. In this study, growth of creeping bentgrass was ceased by herbicide Roundup Pro. A week later, grass was clipped down to thatch layer and covered by a black plastic sheet to avoid stimulation of re-growth. As discussed in the previous study, laccase and guaiacol were applied directly on thatch once every two weeks. However, in this study laccase enzyme was applied at 0, 2.06, and 20.6 units cm-2 with the 10-mL solution of guaiacol at 0 and 0.1 M concentration. Samples were analyzed after two and six months of treatment application. After six months, all treatments with or without guaiacol significantly impacted thatch layer characteristics. Organic matter content in that top 2.5 cm reduced by 24.7% and thatch layer thickness reduced by 57.2% compared to control with laccase treatment of 20.6 units cm-2 (Figure 2). A significant increase in saturated hydraulic conductivity was observed with laccase treatments. A significant reduction in monosaccharide components of structural cellulose and hemicellulose carbohydrates were observed. This reduction in the sugar content can be attributed to the opening of biomass structure by breaking down of lignin macromolecule. However, increase in both acid-soluble and -insoluble lignin components were observed. This increase in lignin components in thatch can be attributed to the over all reduction in structural carbohydrate (sugar) content where lignin and structural carbohydrates are the major components of plant cell wall. Lignin content is calculated as a percent of total lignin and carbohydrates and is thereby dependent on the carbohydrate content. This study supported the original assumption of using lignin degrading enzyme.

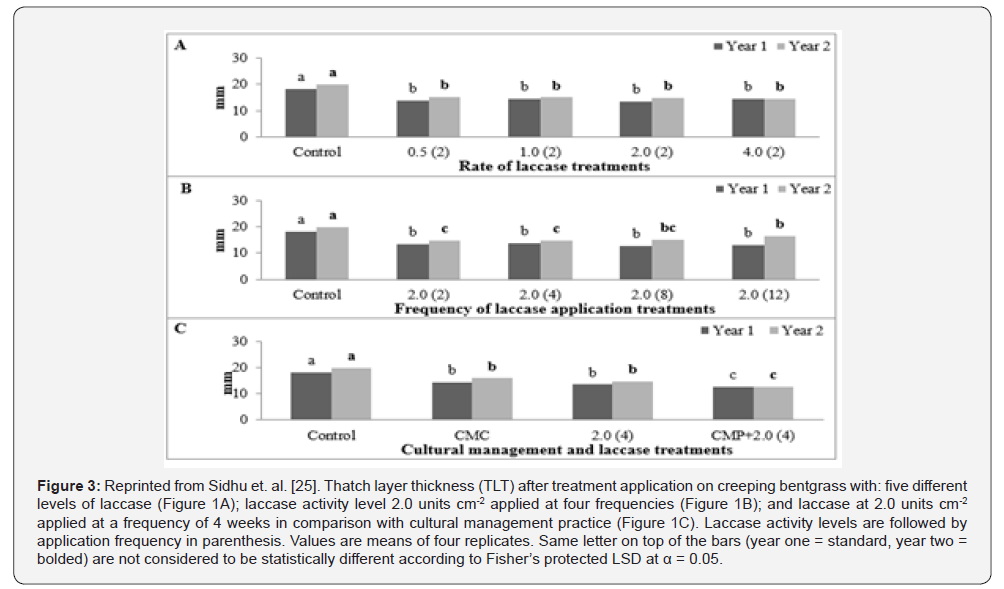
Optimizing laccase application and comparison with cultural practices [25]
In the previous greenhouse study [24], it was evident that laccase, when applied at the activity level of 2.0 units cm-2 once every two weeks, was effective in reducing organic matter buildup and reducing thatch layer thickness. A two-year field study was conducted on creeping bentgrass to evaluate the effectiveness of laccase under field conditions and to optimize the rate and frequency of application. Laccase application was also compared with prevalent cultural practice used in industry, core-aeration followed by sand topdressing. Laccase enzyme was applied as a 410-mL solution over an area of 0.185 m2 for all the treatments.
Rate of laccase application
Laccase was applied at five activity levels 0 (control), 0.5, 1.0, 2.0, and 4.0 units cm-2 at an application frequency of once every two weeks. It was observed that all the levels of laccase treatments were effective in in reducing thatch layer thickness (Figure 3), organic matter content, and carbohydrate content in comparison to control. Lignin content decrease for two lower activity levels of laccase but increased for the higher two levels when compared to control. This increase in the lignin content in higher two levels could be attributed to a higher reduction in carbohydrates in these treatments. The study suggested that laccase application as low as 0.5 units cm-2 is as effective in managing thatch when applied once every two weeks when compared to 2.0 units cm-2 treatment suggested by the greenhouse studies.
Frequency of laccase application
The effective rate of laccase from the previous greenhouse study, 2.0 units cm-2 was applied at four different application frequencies. The application frequencies were once every 2, 4, 8, and 12 weeks. Laccase applied at all application frequencies was effective in managing thatch layer but it was observed that effectiveness of laccase was significantly higher when it was applied once every 2 and 4 weeks as compared to once every 8 and 12 weeks (Figure 3). It was concluded that one application of laccase at activity level of 2.0 units cm-2 once every 4 weeks may be sufficient for efficient reduction in thatch layer.
Laccase in comparison and in combination with cultural practice
Plots that received cultural management treatments were core-aerated and sand top dressed twice yearly. Core-aeration was accomplished with 1.27 cm tines spaced at 5.0 by 5.0 cm. Immediately after core-aeration plots were top dressed with 1134 g of sand. Laccase applications associated with cultural management treatments were applied at 2.0 units cm-2 applied every 4 weeks. Reduction in thatch layer thickness similar in cultural management and laccase treatments (Figure 3). However, a further reduction in thatch layer was observed when laccase was applied in combination with cultural management treatment (Figure 3). Application of laccase in combination with core-aeration and sand topdressing may lead to a reduction in number of cultivations necessary to keep thatch layer at desired levels.
Experimental design limitations
This study was not a factorial design and did not provide the information for a single minimum effective laccase treatment in terms of rate of laccase activity and frequency of application. This was due to the great demand on the available resources. Information gained from this study was later used to design a much smaller factorial experiment to provide minimum effective rate and frequency of laccase application. The results from this factorial study are not published yet.
Impact of laccase on thatch layer of different turfgrass species [26]
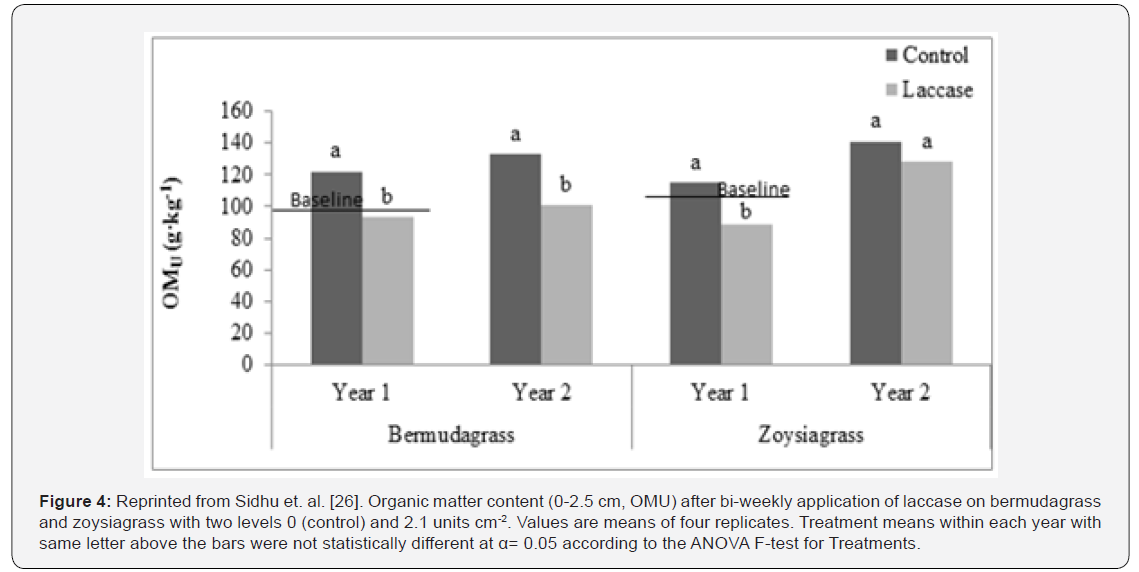
A two year study was conducted on ultra-dwarf bermudagrass (Cynodon dactylon L., ‘TifEagle’) green, and zoysiagrass (Zoysia japonica Stued., ‘Meyer’) maintained as a home lawn to observe the influence of laccase enzyme applications on thatch development. Laccase solution was applied bi-weekly at the activity levels of 0 (control) and 2.0 units cm-2. Response to laccase enzyme applications by both turfgrass species was recorded by measuring physical and chemical properties of thatch layer after six months of treatment applications within each year. A significant 18-22% and 21-30% reduction in thatch layer thickness was observed for bermudagrass and zoysiagrass, respectively. Organic matter content (0-2.5 cm) decreased by 23-24% (Figure 4), while saturated hydraulic conductivity increased by 19-30% for bermudagrass in both years. Acid-soluble and-insoluble lignin reduced in both the grass species after laccase treatments. The results indicate that bi-weekly application of laccase on bermudagrass and zoysiagrass has positive impact on thatch management.
Residual effects of laccase application [27]
A field experiment on creeping bentgrass was conducted to evaluate the residual effects on thatch accumulation after ceasing laccase applications. A significant reduction in thatch layer thickness was observed at 6, 12, and 18 months after treatment initiation when laccase was applied at different rates and frequencies for six months. Residual effects of laccase application were observed as a reduction in thatch layer thickness and no additional accumulation of thatch after six months of treatment cessation. At 18 months after treatment initiation, a significant increase in thatch layer was observed in plots where treatments had ceased for 12 months. This study suggested that either 12 applications of laccase at activity level 0.5 units cm-2 applied once every two weeks (six months) or six applications of laccase at 2.0 units cm-2 applied once every 4 weeks (six months) will be sufficient for managing thatch for a year.
Conclusion
There are several evidences from literature that support the efficacy of laccase enzyme from Trametes versicolor L. to provide sustainable thatch management in turfgrass systems. Laccase when applied at certain rates of activity level and at certain frequency of application for six months in a year is sufficient for thatch control. Future research is needed to further minimize the amount of laccase used, either in terms of laccase activity or in terms of frequency of application to help reduce the cost and number of application treatments per year for this non-destructive thatch management approach.
References
- Ledeboer FB, Skogley CR (1967) Investigations into the nature of thatch and methods for its decomposition. Agron J 59: 320-323.
- Alder E (1977) Lignin chemistry - past, present and future. Wood Sci Technol 11(3): 169-218.
- Del Rio JC, Marques G, Rencoret J, Martinez AT, Gutierrez A (2007) Occurrence of naturally acetylated lignin units. J Agric Food Chem 55(14): 5461-5466.
- Davin LB, Lewis NG (2003) A historical perspective on lignin biosynthesis: Monolignol, allylphenol and hydroxycinnamic acid coupling and downstream metabolism. Phytochem Rev 2: 257-288.
- Chen YR, Sarkanen S (2003) Macromolecular lignin replication: A mechanistic working hypothesis. Phytochem Rev 2(3): 235-255.
- McCarty LB, Gregg MF, Toler JE (2007) Thatch and mat management in an established creeping bentgrass green. Agron J 99: 1530-1537.
- McCarty LB (2005) Best golf course management practices (2nd edn). Prentice Hall Inc. Upper Saddle River, NJ, USA.
- Blanchette RA (1984) Screening wood decayed by white-rot fungi for preferential lignin degradation. Appl Environ Microbiol 48: 647-653.
- Farrell L (1987) Combustion: The microbial degradation of lignin. Annu Rev Microbiol 41: 465-505.
- Baldrian P (2006) Fungal laccases-occurrence and properties. FEMS Microbiol Rev 30(2): 215-242.
- Brunow G (2001) Methods to reveal the structure of lignin. In: Hofrichter M, et al. (Eds), Lignin, humic substances and coal, Biopolymers, (V-I), Wiley-VCH, Weinheim, Germany, UK.
- Higuchi T (2006) Look back over the studies of lignin biochemistry. J Wood Sci 52(1): 2-8.
- Thurston CF (1994) The structure and function of fungal laccases. Microbiol 140: 19-16.
- Gianfreda L, Xu F, Bollag JM (1999) Laccases: a useful group of oxidoreductase enzymes. Biorem J 3: 1-25.
- Singh R, Sidhu SS, Zhang H, Huang Q (2015) Removal of sulfadimethoxine in soil mediated by extracellular oxidoreductases. Environ Sci Poll Res 22(21): 16868-16874.
- Singh R, Cabrera ML, Radcliffe DE, Zhang H, Huang Q (2015) Laccase mediated transformation of 17β-estradiol in soil. Environ Poll 197: 28- 35.
- Ten Have R, Teunissen PJM (2001) Oxidative mechanisms involved in lignin degradation by white-rot Fungi. Chemical Rev 101(11): 3397- 3414.
- Bourbonnais R, Paice MG (1992) Demethylation and delignification of kraft pulp by Trametes versicolor laccase in the presence of 2,2′-azinobis-( 3-ethylbenzthiazoline-6-sulphonate). Appl Microbiol Biotechnol 36(6): 823-827.
- Bourbonnais R, Paice MG, Freiermuth B, Bodie E, Borneman S (1997) Reactivities of various mediators and laccases with kraft pulp and lignin model compounds. Appl Environ Microbiol 63(12): 4627-32.
- Fabbrini M, Galli C, Gentili P (2002) Comparing the catalytic efficiency of some mediators of laccase. J Mol Catal B-Enzym 16(5-6): 231-240.
- Huang Q, Sidhu SS, Raymer PL, Carrow RN (2014) Methods and compositions using fungal laccases to reduce turf thatch. USA.
- Huang Q, Sidhu SS, Raymer PL, Carrow RN (2018) Methods and compositions using fungal laccases to reduce turf thatch. U.S.A.
- Sidhu SS, Huang Q, Carrow RN, Raymer PL (2012) Use of fungal laccases to facilitate biodethatching: A new approach. Hortscience 47: 1536-1542.
- Sidhu SS, Huang Q, Carrow RN, Raymer PL (2013) Laccase mediated changes in physical and chemical composition properties of thatch layer in creeping bentgrass (Agrostis stolonifera L.). Soil Biol Biochem 64: 48-56.
- Sidhu SS, Huang Q, Carrow RN, Raymer PL (2014) Optimizing laccase application on creeping bentgrass (Agrostis stolonifera L.) to facilitate biodethatching. Crop Sci 54: 1804-1815.
- Sidhu SS, Huang Q, Carrow RN, Raymer PL (2013) Efficacy of fungal laccase to facilitate biodethatching in bermudagrass and zoysiagrass. Agron J 105: 1247-1252.
- Sidhu SS (2012) Enzymatic removal of lignin from plant materials: Potential applications. Ph.D. diss., The Univ. of Georgia, Athens, USA.






























