Impact of Commonly Prescribed Antibiotics on Preterm Gut Microbiome in Necrotising Enterocolitis and Late Onset Sepsis
Abdulkadir B1, Abdullahi S2, Abdullahi B3 and Owuna G4
1Department of Microbiology, Umaru Musa Yaradua University Katsina, Nigeria
2Department of Pharmacology, Faculty of Medicine Umaru Musa Yaradua University Katsina, Nigeria
3Department of Microbiology, Ahmadu Bello University Zaria, Nigeria
4Department of Microbiology, Nassarawa State University Keffi, Nigeria
Submission: January 11, 2019; Published: January 24, 2019
*Corresponding author: Abdulkadir B, Department of Microbiology, Faculty of Natural and Applied sciences, Ummaru Musa Yar’adua University P.M.B. 2218, Katsina, Nigeria
How to cite this article: Abdulkadir B, Abdullahi S, Abdullahi B, Owuna G. Impact of Commonly Prescribed Antibiotics on Preterm Gut Microbiome in Necrotising Enterocolitis and Late Onset Sepsis. Curr Trends Biomedical Eng & Biosci. 2019; 18(1): 555979. DOI: 10.19080/CTBEB.2019.18.555979
Abstract
Background: Antibiotics are usually prescribed to preterm infants during their early days of life in neonatal intensive care units. The effects of this intervention on the developing gut microbiome are poorly understood but might have important consequences for health. We aimed to explore the routinely used antibiotics in a neonatal intensive care unit and to what extent this intervention alters the preterm gut microbiome.
Methods: The three most commonly prescribed antibiotic combinations were analysed VCM (Vancomycin, Ceftazidine and Metronidazole), VC (Vancomycin and Ceftazidine) and AFG (Amoxicillin, Flucloxacillin, and Gentamicin). Sampling was performed at four time points: 2-3 days before course started (Pre), last day of administration (During), 1-2 days after antibiotic was given (After), and one week later than or as late as possible before next antibiotic course. In total, 141 stool samples were collected from 38 patients and bacterial profiling was performed by 16SrRNA gene sequencing (Miseq, Illumina)
Results: Bacterial diversity increased significantly after the VC course was stopped (P=0.1). Diversity was reduced for all antibiotic treatment during their administration (P>0.05). Generally, VCM and VC were comparable with lower bacterial taxa when compared to AFG which recorded higher bacterial taxa. The result also showed that VC and VCM recovered but AFG does not.
Conclusion: The three antibiotics courses differentially affected the preterm gut microbiome, causing reductions in the diversity. Further work is necessary to determine the contribution of these changes to health and how medical intervention can be tailored to achieve optimal outcomes for preterm infants.
Keywords: Cellular metabolism; Nutrition; Donor breast milk; Antibiotic
Abbrevations: NICU: Neonatal Intensive Care Unit; VCM: Vancomycin, Ceftazidine and Metronidazole; VC: Vancomycin and Ceftazidine; AFG: Amoxicillin, Flucloxacillin, and Gentamicin
Introduction
The preterm gut microbiome is known to be important in health i.e. cellular metabolism, nutrition and protection and in disease i.e. obesity, diabetes and inflammation [1,2]. As we continue to understand the factors associated with health and disease, we can begin to determine how clinical practise can be tailored to improve the growth and development of preterm infants. Such research includes exploring how different feed (e.g. maternal breast milk, donor breast milk, or formula) affects the developing gut microbiota and modulates the host-microbial interaction in the gut. A study demonstrated how enteral diets and microbial interventions influence the intestinal microbial colonisation [3]. It has been recently reported that antibiotic therapy during pregnancy influence the gut microbial colonisation of maternal mothers and infants [4]. Coupled to this is determining how supplementation can tailor the gut microbiome to maximise health in preterm infants i.e. lactoferin, probiotics [5].
An area which is currently underexplored but of great important to understand how antibiotics intervention in preterm infants, affects the developing gut microbiome, both the immediate and longer-term effects. Antibiotics are natural or synthetic compounds prescribed to extremely preterm infants from birth, usually for the first 48 hours of life without signs of suggestive infection. Thereafter antibiotics are given as intervention when infants present with certain symptoms of complication. The exact antibiotics or combinations of antibiotics administered will vary based on neonatal intensive care unit (NICU). Overall, routine administration of antibiotics to preterm infants likely influences the composition and diversity of the gut microbiota, with important decreases in the number of Bifidobacterium and Bacteriodes, as well as decreased overall diversity [6-9].
Existing research exploring antibiotics in a neonate’s population has shown that early exposure to antibiotics by preterm infants was associated with increased incidence of morbidity and mortality, although this likely a necessity to give antibiotics to the most at risk infants [10]. Antibiotics usage has also been linked to dysbiosis and the subsequent development of Necrotising enterocolitis in the gut of preterm infants [11]. To further explore the effects of antibiotics in preterm infants. We analysed three different antibiotic combinations, which were the most frequently prescribed in the NICU: VCM (Vancomycin, Ceftazidine and Metronidazole), VC (Vancomycin and Ceftazidine) and AFG (Amoxicillin, Flucloxacillin, and Gentamicin). The addition of Metronidazole, which targets anaerobic bacteria, is common in the treatment of severe complication, such as NEC. Metronidazole has previously been shown to alter the rat intestinal microbial community by significantly increasing the number of Bifidobacterium and Enterobacteria [12]. The aim of this study was to explore the effects of routinely prescribed antibiotics in neonatal intensive care units and how it affects the preterm gut microbiome. We hypothesise that distinct changes in the gut microbiota will occur as a direct result of antibiotic administration, which may have important consequences for health.
Methods
Ethical approval
Ethical approval was obtained from the County Durham and Tees Valley Research Ethics Committee. The consent form was duly processed and endorsed by parents for sample collection.
Study design
All the preterm infants in the cohort study were cared for in the neonatal intensive care unit (NICU) of the Royal Victoria Infirmary Newcastle upon Tyne. There is a standard feeding practice in the unit; antibiotics, antifungal as well as probiotics were used depending on the clinical diagnosis. All the infants in the cohort were <30 weeks gestational age and <1500g birth weight with the exception of patients 207 having 1580g and patient 294 with 1650g (Supplementary Table 1), and were grouped in to “pre” (2-3 days before administration), “during” (last day of administration or within 2 days of antibiotic course ending), “after 1” (1-2 days after last antibiotic), and “after 2” (1 week after last antibiotic) (Table 1). Overall, 141 stool samples and clinical data were collected from 38 infants, out of which 12 preterm infants received VCM (41 samples), 13 VC (51 samples) and 13 (49 samples) AFG course.
Sample analysis

NGS 16S rRNA gene bacterial profiling was performed on all samples in the study. Total nucleic acid extraction was performed from 100 mg of stool using the PowerLyzer™ Power Soil® DNA Isolation Kit (MoBio) following the manufacturer’s guidelines. Bacterial profiling utilised the 16S rRNA gene targeting variable region 4 and was carried out by ‘NU-OMICS’ (Northumbria University) based on the Schloss wet-lab MiSeq SOP and resulting raw fast data were processed using Mothur (version 1.31.2) as described previously [13]. Briefly, combined reads were trimmed to 275 reads with 0 ambiguous bases. Chimeric sequences were detected by Chimera. uchime and removed from downstream analysis. Alignment was generated via the Silva v4 database [14] and Chloroplast, Mitochondria, unknown, Archaea, and Eukaryota linages were removed from the analysis. In total, 12,079,417 reads were obtained, and sequences were deposited in MG-RAST under its corresponding accession numbers. The data was subsampled to 7,500 reads per sample using the sub. sample command in Mothur (Table 1).
Results
Figure 1 above indicates the average OTUs of the highest relative abundance OTUs and the effect of antibiotic treatment to microbial community in the cohort study, A, B & C represents VCM, VC & AFG respectively. Proportion of OTUs matching the 5 most frequently observed taxa over time among the antibiotic course. Colours on the charts matching with the bacterial taxa on the legend represent the diversity in percentages. While the letters I, II, III and IV on the bottom of each chart representing different sampling points of the antibiotics I, II, III and IV represents pre, during, after and 1 week after respectively.
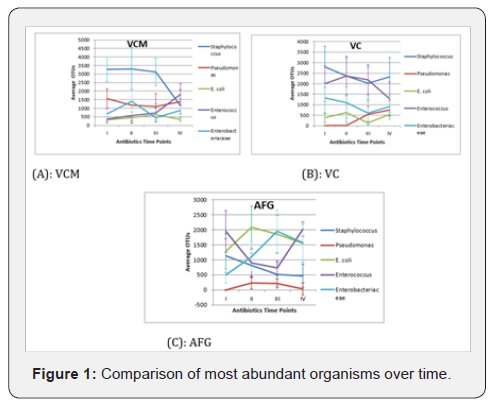
We observed that Staphylococcus had a significantly higher (P=0.004) number of OTU in the VCM treatment across the early time points but this was reduced 1 week after treatment stopped and showed no significant difference (P=0.157) compared to other taxa except with E. coli (P = 0.006) (Fig. 1-A). However, the other taxa (Pseudomonas, Enterococcus and Enterobactereaceae) increased at 1 week after VCM regimen stopped but not reached significantly so (Figure 1A). Staphylococcus and Enterococcus OTUs were high in the VC regimen across all the time points when compared to other taxa (Figure 1B). All taxa increased at 1 week after VC regimen was stopped except Enterococcus, (Figure 1B). Pseudomonas OTUs were lower in AFG treatment across the time points when compared to other taxa (Figure 1B). All taxa have stable OTUs in AFG treatment at 1 week after treatment stopped except Enterococcus OTUs that increased significantly (P = 0.02) (Figure 1C). The other most abundant taxa showed inconsistent patterns across the time points between the different the entire antibiotic courses.
Generally, Staphylococcus was the most abundant taxa with high OTUs in almost all the antibiotic mixtures in the cohort also there is overall increase in OTUs number with antibiotic treatment at 1 week after regimens were stopped with the exception of Staphylococcus (in VCM treatment) and Enterococcus (in VC treatment). The letters below on the X axis represent time points; where I = pre, II = during, III = after, IV-1 = 1 week after and IV-2 = more than 1 week before next antibiotic course. Asterisks represent the outliers, horizontal lines in the boxes represent the median value, shade boxes indicate upper and lower quartiles and the vertical lines extending from the boxes represent highest and lower whiskers. Diversities not significantly different between time points (P = 0.3) (Figure 2A).
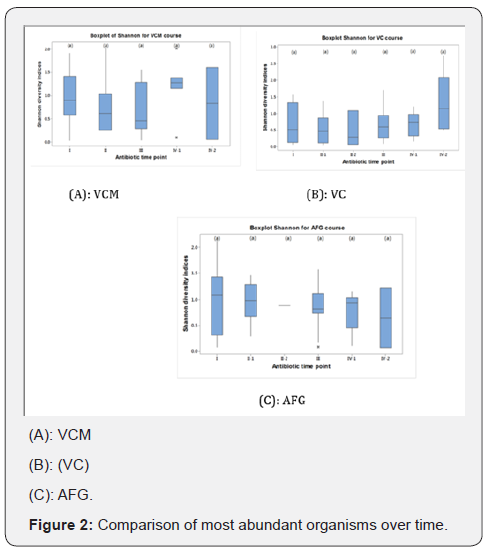
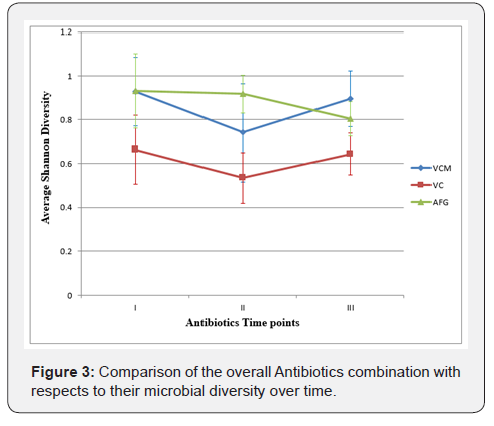
From Figure 2B, the letters below on the X axis represent time points; where I = pre, II-1 = 1-2 day during antibiotics administration, III = after, IV-1 = 1 week after and IV-2 = more than 1 week before next antibiotic course. The horizontal lines in the boxes represent the median value, shade boxes indicate upper and lower quartiles and the vertical lines extending from the boxes represent highest and lower whiskers. Diversities were not significantly different between time points (P- value 0.1). Figure 2C the letters below on the X axis represent time points; where I = pre, II-1 = 1-2 day during antibiotics administration, III = after, IV-1 = 1 week after and IV-2 = more than 1 week before next antibiotic course. *Asterisks represent the outliers, horizontal lines in the boxes represent the median value, shade boxes indicate upper and lower quartiles and the vertical lines extending from the boxes represent highest and lower whiskers. Diversities are not significantly different between time points (P- value 0.9). Although, there was a general trend of decreased diversity, this was not significant between treatment time points for any antibiotic mixture (Figure 2). Overall average diversity between antibiotic mixtures was calculated, the mean, standard deviation and mean error was also calculated (Figure 3). A comparable pattern was observed between VCM and VC treatment across the time points with VC lower in diversity than VCM and AFG (Figure 3). Generally, there is decrease in microbial diversity with all treatment during antibiotics administration and increase after the treatment was stopped except in AFG regimen (Figure 3).
Discussion
We robustly explored faecal samples derived from 38 premature infants by subjecting them to 16S rRNA sequencing and further statistical analysis. We compared between three antibiotic regimens which including one incorporating metronidazole to look at their impact on bacterial community in the gut of these infants. Our hypothesis was that certain antibiotic regimen may alter the preterm gut microbiota.
Overall comparison between antibiotic courses: The result from average Shannon diversity showed the following distinct points:
i. There is general decrease in microbial diversity across the entire course during the antibiotics administration and increase after the treatment stopped except in AFG, but these differences are not significant (P = 0.9).
ii. The overall response of the gut bacterial communities showed comparable patterns between VCM and VC regimen. However, the VC regimen had a lower diversity, but not significantly so (P = 0.1).
iii. The, AFG regimen had a higher diversity at sampling points when compared to other treatments, but this decreased after treatment was stopped though we found no statistical difference between the means (P = 0.5) (Figure 3).
iv. The overall result showed the recovery of microbiome after VCM and VC regimens stopped but AFG does not (Figure 3).
Moreover, with regards to the most abundant taxa, Staphylococcus had a high OTU number in VCM and VC regimens across the sampling points with a decrease one week after VCM treatment was terminated. Enterococcus also decreased in VC one week after treatment but with no significant difference (P = 0.181) when compared with other abundant taxa. Pseudomonas was lower in AFG regimen with a significant difference between its mean value and that of Enterococcus, E. coli and Enterobactereaceae across the time points (P = 0.02). Staphylococcus was significantly different when compared with Pseudomonas (P = 0.05) at point 1, but statistically not different at remaining sampling points (P = 0.27) (Figure 3).
Our findings are closely related to previous study which observed a significant reduction in the diversity of bacterial populations within the gut of preterm infants [10]. That study showed a reduction in bacterial diversity during antibiotic intake with an increased contribution of Enterobacter in to the community [10]. Other studies have reported low microbial diversity with an increase in Proteobacteria in the preterm infants associated with NEC [15], and that the abundance of Bifidobacterium in the gut is reduced as a result of antibiotic usage which may result in dysbiosis [16]. Similarly, the effect of antibiotics treatment in reducing the bacterial diversity as well as that of Bifidobacterium and Bacteriodes among infants of less than 12 month of age [7] has been reported. It has also been shown that the pattern of bacterial colonisation after one week of antibiotics treatment resemble that of at the initial point of administration [17].
Furthermore, our research demonstrated how certain antibiotics combinations altered the composition and structure of the bacterial communities from the gut of preterm infants during the early days of life (Table 1). This supports with work reporting the use of antibiotics course combination within the 48 hrs of birth among preterm infants significantly altered the gut microbial community [18]. Previous work has demonstrated that the intestinal microbiome of infants who received antibiotics treatment had high percentage of Proteobacteria and lower percentages of Actinobacteria, Bifidobacterium and Lactobacillus than infants that did not, even after the antibiotic dose was stopped. However, two month later, Actinonobacteria, Bifidobacterium and Lactobacillus recovered to levels observed before the antibiotic treatment [18]. Similarly, a commensal microbiome in the human gut were shown to stabilise a few weeks after antibiotics treatment ceased [19].
During our study, we detected some bacterial taxa (Figure 1 & Table 1) differentially in high abundance prior to antibiotics administration; these might be passed across from the maternal microbiota during birth or acquired immediately after delivery as it is recognised previously that the preterm GIT often harbour a microbiota resembling that of mother’s skin or vaginal community dependent on the mode of delivery [20,21]. However, it has been reported that preterm gut microbiome consists of more pathogens when compared to healthy term infants [10,22,23]. Metronidazole was described as one of the most commonly used antibiotics for the treatment of pathogenic anaerobic bacteria.Its clinical importance relates to it being cheap, its mechanisms of action being against anaerobic infections, that it had less adverse effects than many alternating and ease of application [24]. However, combining it with other antibiotics is clinically important during the therapy of both aerobic and anaerobic infections [24,25]. In our cohort, we observed that when metronidazole was added to the VC course, a distinct bacterial community pattern was found enriched in Staphylococcus with fewer Enterococcus, compared to VC treatment alone. Also, there is significant (P = 0.02) increase in Pseudomonas in VC versus VCM regimen after treatment stops. This signifies that metronidazole when combine with ceftazidine has a great effect against Pseudomonas. Moreover, Staphylococcus and E. coli were reduced in VCM versus VC regimens after treatment stops which imply the effects of Metronidazole against anaerobic bacteria (Table 1). However, in AFG regimen, Staphylococcus decrease across the time points indicating the effect of antibiotics mixture against Staphylococcus as one of the target organisms (Table 1). With regards to Lactobacillus’s, Veillonella, Bifidobacterium, Acinetobacter and Enterobacteriacaea; there is an inconsistent trend across all the sample points during the cohort study which indicates a large amount of variation within individuals in term of their gut bacterial community and how it responds to antibiotic treatment [5].
Microbial diversity: Generally, AFG recorded higher diversity, followed by VCM and then VC with least diversity in the cohort study (supplementary Figure 1). While with respect to sampling points, microbial diversity was higher prior to antibiotics intake and reduced during the antibiotic’s intake in the entire courses with variation after the dose was finished. For VCM and AFG, it was decreased across the time points but increased after VC course was stopped (supplementary Figures 1-3). Though, we noticed the short-term recovery of some bacteria from the preterm gut after antibiotics treatment, it varied and were inconsistent depending on the antibiotic combination. There is limited published data reported on the short-term recovery of microbiome from the infant’s gut after antibiotics therapy [18] although some studies demonstrated both short- and long-term impacts of antibiotics treatment on gut microbiome [19,26] with also few studies on long-term impacts [26]. However, a lack of a definite pattern of bacterial recovery after antibiotics treatment has also been reported [7].
Our study involved cross sectional samples from preterm infants receiving different antibiotics combinations. However, the limitations of our study include the low number of the preterm infants who received different antibiotics regimen and the limited antibiotics combination that could be studied. In future, it will be necessary to explore more antibiotic regimen in larger cohorts and compare that data with the present study.
Conclusion
Generally, there is decrease in microbial diversity during antibiotics intake for the entire course and an increased at one week after the treatment terminated in all the antibiotic mixture with the exception of AFG regimen. VCM and VC showed similar pattern of microbial diversity across the time points. We therefore conclude that antibiotics administration may alter the microbial diversity from the gut of preterm infants. Further study is necessary on other antibiotic regimens that are routinely given to preterm infants and their clinical impacts in health and disease as our study is limited to particular course due to unavailability of the desired samples from other combinations.
References
- Inna S, Brett FB, Shannon LR (2010) Gut Microbiota in Health and Disease. Physiol Rev 90(3): 859-904.
- Stewart CJ, Marrs ECL, Nelson A, Lanyon C, Perry JD, et al. (2013) Development of the Preterm Gut Microbiome in Twins at Risk of Necrotising Enterocolitis and Sepsis. PLoS One 8(8): e73465.
- Cilieborg MS, Boye M, Sangild PT (2012) Bacterial colonization and gut development in preterm neonates. Early Hum Dev 88(Suppl 1): S41- 49.
- Gonzalez Perez G, Hicks AL, Tekieli TM, Radens CM, Williams BL, et al. (2016) Maternal Antibiotic Treatment Impacts Development of the Neonatal Intestinal Microbiome and Antiviral Immunity. J Immunol 196(9): 3768-3778.
- Berrington EJ, Christopher JS, Stephen PC, Nicholas DE (2014) The neonatal bowel microbiome in health and infection. Curr Opin Infect Dis 27(3): 236-243.
- John P, Carel T, Cornelis V, Bianca S, Ischa K, et al. (2006) Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 118(2): 511-521.
- Johnson CL, Versalovic J (2012) The human microbiome and its potential importance to pediatrics. Pediatrics 129(5): 950-960.
- Roberto Murgas Torrazza MD, Josef Neu M (2012) NIH Public Access. Clinical Perinatol 40(1): 93-108.
- Ward Doyle V, Dirk Gevers, David S Newburg, Ardyth L Morrow (2014) Impact of Antibiotic administration on the establishment and development of infant Gut flora. Infants Gut Metagenomics Initiative.
- Greenwood C, Morrow AL, Lagomarcino AJ, Altaye M, Taft DH, et al. (2014) Early Empiric Antibiotic Use in Preterm Infants Is Associated with Lower Bacterial Diversity and Higher Relative Abundance of Enterobacter. J Pediatr 165(1):23-29.
- Torrazza RM, Ukhanova M, Wang X, Sharma R, Hudak ML, Neu J, Mai V (2013) Intestinal microbial ecology and environmental factors affecting necrotizing enterocolitis. PloS One 8(12): e83304.
- Pélissier MA, Vasquez N, Balamurugan R, Pereira E, Dossou Yovo F, et al. (2010) Metronidazole effects on microbiota and mucus layer thickness in the rat gut. FEMS Microbiology Ecology 73(3): 601-610.
- Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD (2013) Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol 79(17): 5112-5120.
- Schloss PD, Gevers D, Westcott SL (2011) Reducing the Effects of PCR Amplification and Sequencing Artifacts on 16S rRNA-Based Studies. PLoS One 6(12): e27310.
- Wang Y, Hoenig JD, Malin KJ, Qamar S, Petrof EO, et al. (2009) 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. ISME J 3(8): 944-954.
- Kheadr E, Dabour N, Le Lay C, Lacroix C, Fliss I (2007) Antibiotic susceptibility profile of bifidobacteria as affected by oxgall, acid, and hydrogen peroxide stress. Antimicrob Agents Chemother 51(1): 169- 174.
- Dethlefsen L, Relman DA (2011) Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci U S A 108(Suppl 1): 4554-4561.
- Fouhy F, Guinane CM, Hussey S, Wall R, Ryan CA, et al. (2012a) Highthroughput sequencing reveals the incomplete, short-term recovery of infant gut microbiota following parenteral antibiotic treatment with ampicillin and gentamicin. Antimicrob Agents Chemother 56(11): 5811-5820.
- Jernberg C, Löfmark S, Edlund C, Jansson JK (2010) Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology 156(Pt 11): 3216-3223.
- Mshvildadze M, Neu J, Shuster J, Theriaque D, Li N, Mai V (2010) Intestinal microbial ecology in premature infants assessed with nonculture- based techniques. J Pediatr 156(1): 20-25.
- Nyangale EP, Mottram DS, Gibson GR (2012) Gut microbial activity, implications for health and disease: the potential role of metabolite analysis. J Proteome Res 11(12): 5573-5585.
- Mai V, Young CM, Ukhanova M, Wang X, Sun Y, et al. (2011) Fecal microbiota in premature infants prior to necrotizing enterocolitis. PLoS One 6(6): e20647.
- Morowitz MJ, Denef VJ, Costello EK, Thomas BC, Poroyko V, et al. (2011) Strain-resolved community genomic analysis of gut microbial colonization in a premature infant. Proc Natl Acad Sci U S A 108(3): 1128-1133.
- Löfmark S, Edlund C, Nord CE (2010) Metronidazole is still the drug of choice for treatment of anaerobic infections. Clin Infect Dis 50(Suppl 1): S16-23.
- Zar FA, Bakkanagari SR, Moorthi KMLST, Davis MB (2007) A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis 45(3):302-307.
- Jernberg C, Löfmark S, Edlund C, Jansson JK (2007) Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J 1(1): 56-66.






























