Hepatocurative and Gluco-stabilizing Potentials of Ethanol Extract of Stem bark of Flacourtia indica in Aluminium Chloride induced Toxicity in Albino Wistar rats
Amany M Basuny1, Shaker M Arafat2 and Aboel-Ainin AMH Mostafa1
1Depatmnet of Biochemisrty, Agriculture Beni-Suef University, Egypt
2Department of Oils & Fats Research, Food Technology Research Institute, Egypt
Submission: November 23, 2018; Published: January 18, 2019
*Corresponding author: Amany M Basuny, Biochemistry Department, Faculty of Agriculture Beni-Suef University, Oils & Fats Research Department, Food Technology Research Institute, Agricultural Research Center, Egypt
How to cite this article:Amany M B, Shaker M A, Aboel-A AMH M. Amany M Basuny1, Shaker M Arafat2 and Aboel-Ainin AMH Mostafa1. Curr Trends Biomedical Eng & Biosci. 2019; 18(1): 555976. DOI: 10.19080/CTBEB.2019.18.555976
Abstract
The changes occurring in low acidity rice bran oil and its blend with palm olein during repeated frying cycles at 180˚C±5˚C for 4 hours a day up to five days of potato chips were determined. The parameters assessed were: acidity, color, smoke point, peroxide value, polar content, polymer content, oxidative stability by Rancimat method, tocopherol content, oryzanol content and fatty acid composition of rice bran oil, palm olein and binary oil mixtures were evaluated. Results indicated that, rice bran oil better stability than the palm olein in deep frying of potato chips. In general, this finding provides useful information to food processors and consumers who stable and healthy for frying process.
Keywords: Rice bran oil; Deep-fat frying; Palm olein; Oxidative stability
Introduction
Frying is an ancient and well-established process in food preparation. This is evidenced by a great increase in fried food consumption in the recent years, of which more than 20 million tones of world annual oil production is extensively utilized for frying [1]. The process is essentially a dehydration of food that involves rapid heat and mass transfer in food immersed in hot oil at temperatures greater than the boiling point of water [2]. During frying, oils are degraded from thermal oxidation to form volatile and non-volatile decomposition products [3]. The chemical changes in frying oil also result in changes in the quality of fried food. The fatty acids composition of the frying oil is an important factor affecting fried food flavor and its stability; therefore, it should be low level of polyunsaturated fatty acids such as linoleic or linolenic acids and high level of oleic acid with moderate amounts of saturated fatty acids [4,5].
As a result, the quality of frying oil is important because of absorbed oil of fried products during deep-fat frying process. Palm oil, particularly its liquid fraction palm olein, is extensively used in frying sectors. The oil is normally regarded as heavy duty oil because of its technoeconomic advantages over other vegetable oils; this can be explained from the basis of its stronger heat resistance and competitive trading price [6]. Despite all this, the prospect of palm olein seems not convincing enough to some food processors and consumers not only because of perceptions on issues associated with saturated oils but also related to the preference of their local oils. Realizing the limitation of unsaturated oils in the perspective of stability, one of the routes to penetrate the use of palm olein, particularly for industrial frying, is through blending. Frying stability of palm olein blends is widely discussed [7-9].
Rice bran oil is one of the most nutritious and healthful edible oils due to the presence of abundance natural bioactive phytoceuticals such as γ-oryzanol, tocopherols, tocotrienols (tocols) and play important roles in preventing some diseases [10]. Rice bran oil has about 30% linoleic acid, 44% oleic acid and about 23% saturated fatty acids. Unsaturated are susceptible to oxidation or thermal degradation during heating or frying [11], leading to various chemical changes such as oxidation, polymerization, pyrolysis and hydrolysis [12].
Rice bran oil is popular to be used as frying oil due to its high smoke point, low viscosity and high stability and unique frying characteristics which required less oil in frying compared to other oils [13]. Rice bran oil plays important role as excellent salad and frying oil with high oxidative stability resulting from its high level of tocopherols and tocotrienols [13]. The objective of this study was designed to investigate the effect of deep-fat frying on physico-chemical properties of rice bran oil extracted from heating process of rice at 100˚C for 6 minutes [14] and blends. Also, determine of γ-oryzanol and α-tocopherols in oil samples. And further provide an alternative heating oil source as major commercial vegetable oils in deep-fat frying process.
Materials and Methods
Source of Oils
Low acidity rice bran oil was obtained from heating process of rice at 100˚C for 6min according to Arafat et al. [15]. And palmolein for comparison in the frying was purchased from a local supermarket.
Preparation of Rice Bran Oil Blends
The rice bran oil blends were prepared by: (1) 100% rice bran oil, (2) 100% palm olein, (3) 70% rice bran oil and 30% palm olein, (4) 50% rice bran oil and 50% palm olein and (5) 30% rice bran oil and 70% palm olein.
Frying Protocols and Oil Sampling
Frying trials were carried out using a stainless-steel fryingpan. The frying test was carried out at 180˚C±5˚C with the frying time of 20hrs over 5 days. Potatoes were washed, peeled and cut into slices (2mm thickness) previously soaked in NaCl solution (10%w/v) was fried. The weight of chips fried for each batch was about 200gm. The frying process was repeated for five consecutive days for 4hrs every day. At the end of the frying experiment each day, the oil was left to cool overnight. An amount of 400ml frying oil was sampled in 500ml dark bottles, flushed in nitrogen and stored at -18˚C for physic-chemical analysis. All the samples collected were analyzed using the same procedure used for initial oil analysis. All testing and analysis were repeated three times to obtain an average reading.
Quality Assurance Tests for Non-Fried and Fried Oil Blends
Iodine number (Hanus), acid value, and peroxide value were determined according to method of A.O.A.c Guideline [16]. Smoke point refers to the temperature to smoke and is recorded as out lined by Nielson [17]. A Lovibond Tintometer apparatus (The Tintometer Ltd., Salisburg, England) was applied to measure the color of non-fried and fried oil samples. The yellow glass slide was fixed at 35 and the intensity of red glass was assigned through matching with the oil samples [17]. Polymer content for oil samples was determined according to the methods Wu & Nawar [18], respectively. Polar compounds in oil samples were measured by column chromatography according to the method described by Waltaking & Wessels [19].
Estimation of Tocopherols
The content of tocopherols was analyzed in high performance liquid chromatography (HPLC) system (Gilson Inc., Middleton, WT), equipped with a fluorescence detector and an outsampler (Perkin Lmer, Waltham, MA), as described in AOCS Official Ce- 8-89 [20].
Estimation of Oryzanol
Estimation of Oryzanol Antifungal resistanceOryzanol content was estimated given by Seetharamaiah & Prabakar [21]. Accurately weighed oil samples (About 10mg each) in replicates were dissolved in hexane and made up to 10ml. O.D. of the solution was recorded in a 1-centimeter cell at 314nm in a Shimadzu UV-240 double beam recording spectrophotometer (Solutions having OD more than 1.20 were further diluted before recording). The oryzanol content in the oil was calculated using the formula:
Oryzanol, g%= O.D of hexane solution/weight of oil (g)/100ml x 100/ 358.9.
Oxidative stability (Rancimat)
The oxidative stability was estimated by measuring the oxidation induction time, on a Rancimat apparatus (Metrohm CH series 679). Air (20L/h was bubbled through the oil (5.0g) heated at 100 ˚C ± 2 ˚C, with the volatile compounds being collected in water, and the increasing water conductivity continually measured. The time taken to reach the conductivity inflection was recorded Farhoosh [22].
Fatty Acids Composition
Capillary gas chromatograph (HP 6890) was used for the qualitative and quantitative determinations of fatty acids of the oil samples and reported in relative area percentages. Fatty acids were transesterfied into their corresponding fatty acid methyl esters by shaking a solution of oil (0.1g) in heptane (2 ml) with solution methanolic potassium hydroxide (0.2 ml, 2N). The fatty acid methyl esters were identified using a gas chromatograph equipped with DB-23 (5%-cyanopropyl–methyl poly siloxane) capillary column (60mx 0.32mm X0.25μm film thickness) and flame ionization detector. Nitrogen flow rate was 0.6ml/min, hydrogen and air-flow rates were 45 and 450ml/ min, respectively. The oven temperature was isothermally heated 195˚C. The injector and the detector temperatures were 230˚C and 250˚C, respectively. Fatty acid methyl esters were identified by comparing their retention times with known fatty acid standard mixture. Peak areas were automatically computed by an integrator. All GC measurements for each oil sample were made in triplicate and the averages were reported.
Data Evaluation
All experiments and measurements were carried out in triplicate, and the data were suggested to analysis of variance (ANOVA). Analysis of variance and regression analyses were performed according to the MStatC and Excel software. Significant differences between means were determined by Duncan’s multiple range tests. P values less than 0.05 were considered statistically significant.
Results and Discussion
The initial qualities of rice bran oil and palm olein were shown in Table 1. As overall, free fatty acids, peroxide value, iodine value and smoke point of rice bran oil were tested higher than palm olein. The free fatty acids of palm olein at 0.30% were able to meet the standard trading specification of 0.1% free fatty acids maximum. Meanwhile peroxide value of both oils was considered lower than the specification for fresh oil. Iodine value for rice bran oil was much higher due to the higher degree of unsaturation. Smoke point for both oils was higher than 200˚C making these oils were suitable for deep-fat frying purposes.
Fatty acids composition is one of the direct routes to predict the stability of oils. Table 1 shows the fatty acids composition of parent oils that is palm olein, rice bran oil and binary blends. Palm olein contains a balance proportion of saturated and unsaturated fatty acids, and this imparts to the stability of oil. Rice bran oil has palmitic acid (23.00%) as the major saturated fatty acids. It is high in oleic acid (42.00%) and linoleic acid in the largest component (28.40%) of or of polyunsaturated fatty acids, followed by low level (1.10%) linolenic acid.
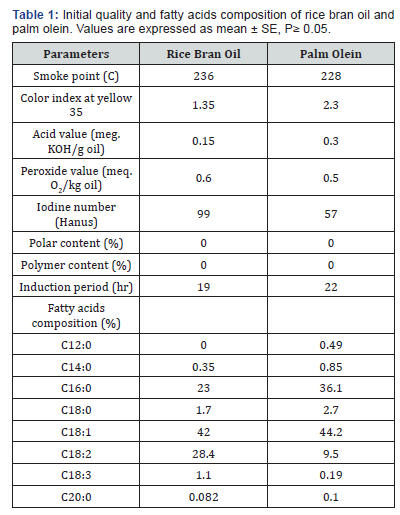
Physico-Chemical Properties of Oil During Frying
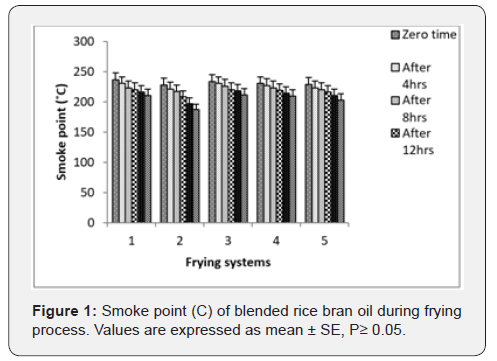
Smoke Point: Smoke point is the temperature of which the oil starts to produce a continuous wisp of bluish smoke when heating takes place. The presence of free fatty acids is generally associated with the smoke point value. This is based on the fact that the amount of smoke emanating from the oil is directly proportional to the concentration of low-molecular-weight constituents, for example, free fatty acids, monoacylglycerols, diacylglycerols and volatile compounds [23]. Changes in smoke point of oil across 5 days of frying operation are illustrated in Figure 1. Values of smoke point of fried rice bran oil were gradually decrease compared with palm olein. It is worth nothing that the smoke point of fried rice bran oil mixed with palm olein at variance levels were generally higher than rice bran alone.

Color: In most cases, two types of colored glasses of Lovibond Tintometer, i.e., yellow and red, were used to measure the color of the oils. The yellow glasses were fixed at value of 35 and the variation in oil color was matched with red glasses. Figure 2 illustrate that the initial red colors for palm olein were 1.90 and 1.80, respectively. As a general trend, the intensity of the red color in all oil systems was increased as the frying time increased. Accordingly blending palm olein with rice bran oil produced lighter frying media.
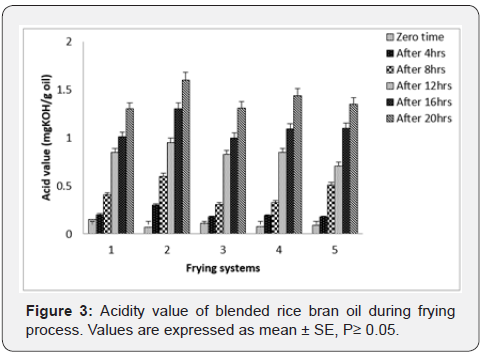
Acidity: Acidity is basically development when oils composition in hydrolytically altered as a result of reaction with moisture release from food, and partly due to decomposition of oils at frying temperatures [24]. Fresh rice bran oil, palm olein and the oil blends were of good quality. Acidity increased after the deep-fat frying cycles but no significant differences (P < 0.05) was observed in the acidity of rice bran oil and blended oil samples between consecutive frying cycles, 1 day to 3 day (Figure 3). Rice bran oil and oil blended samples obtained after 4 day frying cycles had significantly different acidity. Hence, the increase of the acidity was in the order palm olein > palm olein + rice bran oil (30:70) > palm olein + rice bran oil (50:50) > rice bran oil + palm olein (70:30) > rice bran oil.
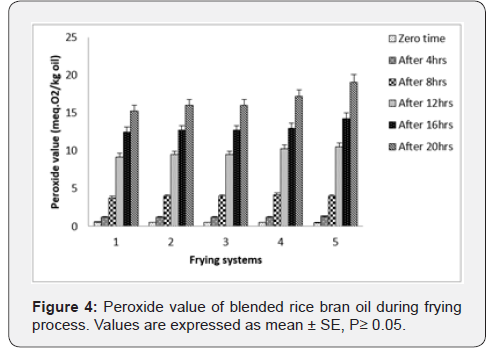
Peroxide Value: Primary oxidation reactions cause an increase in the concentration of peroxides to a maximum value beyond which its concentration decreases due to thermal decomposition therefore into carbonyl compounds and aldehydes [25]. The results given in Figure 4 shows that the peroxide value of rice bran oil and blended during frying at 180˚C±5˚C, which gradually increased from to Meq.O2/kg oil. The values of peroxide value for the oils samples at the end of frying period indicate that the increase of peroxide value was in the order: palm olein > palm olein + rice bran oil (30:70)> palm olein + rice bran oil (50:50) > rice bran oil + palm olein (70:30) > rice bran oil. Slow rate of increase in acidity and peroxide value may be attributed due to the protective effect of oryzanol present in rice bran oil.
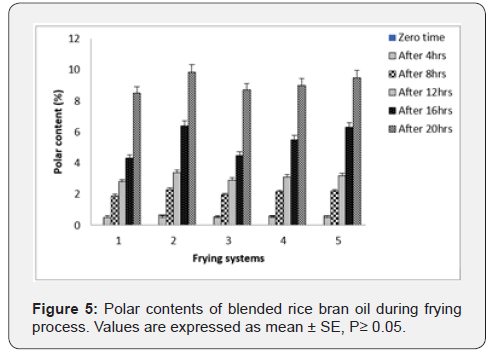
Polar Compounds: Polar compounds are considered as the most objective method to examine the deteriorative effect in frying oils [26]. The polar compounds fractions that is, polymerized and oxidized triacylglycerol and diacylglycerol and free fatty acids are being development during the oxidation and polymerization stages [27]. Changes in polar content of rice bran oil and blended oil are shown in Figure 5. At zero-time, non-detectable polar compounds were found. Frying of rice bran oil at 180 ˚C±5 ˚C for 4hr/5 days caused increased in polar compounds content of all oil systems. The increases of polar compound content of oil systems were in palm olein > palm olein + rice bran oil (70:30) > palm olein + rice bran oil (50:50) > rice bran oil. In addition, blending rice bran oil with palm olein induced lowering effect on the formation of total polar content.
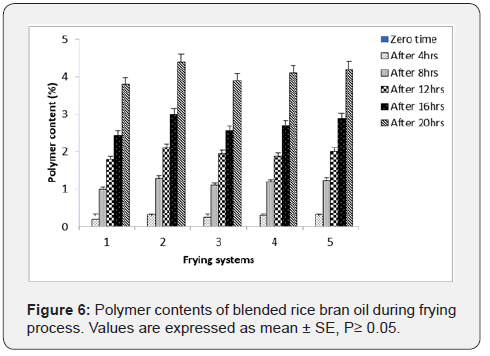
Polymer Compounds: Polymer compounds which are a fraction of polar compounds are developed through tertiary oxidation and thermal modification in oil structure when exposed to high temperature, the latter is more prominent based on the fact that steam release from the product provides some form of protection to the frying oil by minimizing content with oxygen [28]. The formation of polymer compounds is responsible for the change in oil viscosity, tendency to foam during frying and imparts bitterness to the fried product [29]. The development of polymer compounds across throughout the course of frying is shown in Figure 6. At the end of frying the percentage of polymer compounds in palm olein was the greatest, followed by palm olein + rice bran oil (70:30), rice bran oil +palm olein (70:30) and rice bran oil.
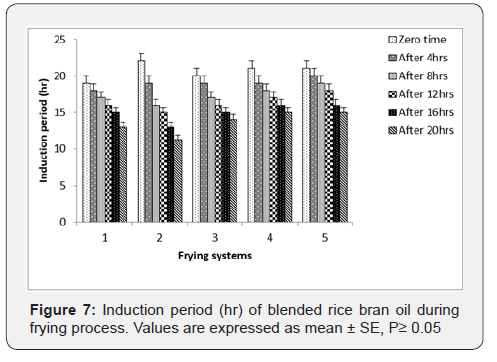
Oxidative Stability: Oxidative stability is an expression to describe the extent of oil stability by examines the time needed for oil to resist oxidation at elevated temperatures [23]. From Figure 7, the oxidative stability of fresh palm olein, rice bran oils and their blends were the highest, Results shown in Figure 7 demonstrate a gradual decrease and significant in the oxidative stability of oil samples during the first stage of frying time process.
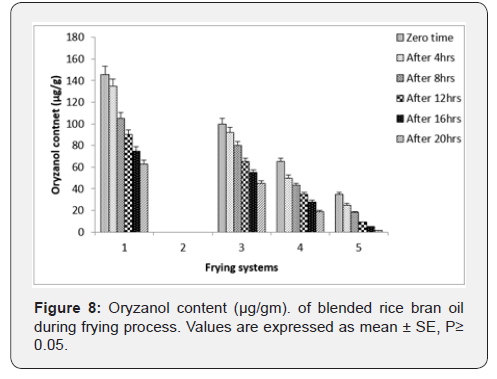
Oryzanol Content: Rice bran oil is known for the oryzanol content present in it and the derived potential benefits. However, it is absent in the palm olein therefore the resultant blended oils from the rice bran oil and palm olein contained lesser oryzanol content. The oryzanol content decreased with the frying time in both the oils, when used as frying media for potato chips. However, the decrease during the frying operation was more prominent in rice bran oil as compared to the blended oils. The oryzanol content reduction was more in rice bran oil, when used to fry the potato chips. Significant changes in the oryzanol content of rice bran oil and blended oil were observed after 3rd frying day (Figure 8) Shin et al. [30] found that oryzanol (Gamma) acts as an antioxidant in the oil but is lost during the thermal oxidation.
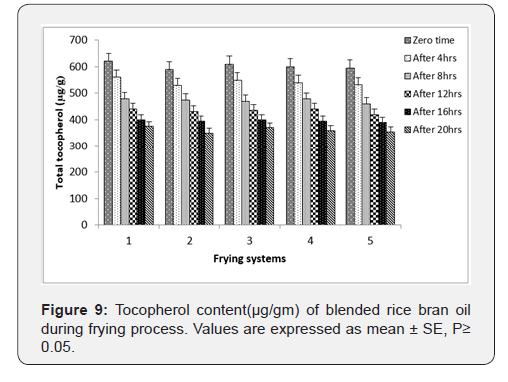
Tocopherol: Tocopherol commonly known as vitamin E are natural antioxidants that inherently present in oils. These vital constituents principally protect the oils by acting as radical scavenger to decelerate the propagation phases of oxidative degradation [31]. The change in tocopherol with the increase in frying times is also presented in Figure 9. All oil samples experienced a gradual drop of tocopherol content during the frying process.
Conclusion
This study examined the physico-chemical changes in oil samples when rice bran oil was blended with palm olein in the form of binary mixtures. This is to improve oil stability before use under continuous frying conditions. Oil samples deterioration was relatively slow across frying times and in most cases, the oxidative stability of the oil blends was equivalent to that of rice bran oil. Indeed, this finding provides useful information to food processors and consumers who are looking for stable and healthy oil for frying process.
References
- Gertz C (2004) Deep frying remains an art. European Journal Lipid Science and Technology 106(11): 713-714.
- Farkas BE, Singh RP, Rumsey TR (1996) Modeling heat and mass transfer in immersion frying: 1-model development. Journal Food Engineering 29(2): 211-226.
- Melton SL, Jafar S, Sykes D, Trigiano MK (1994) Review of stability measurements for frying oils and fried food flavor. Journal of American Oil Chemists Society, 71(12): 1301-1308.
- Kiatsrichart S, Brewer MS, Cadwallder KR, Arty WE (2003) Pan-frying stability of nusun oil, a mid-oleic sunflower oil. Journal of American Oil Chemists Society 80: 479-483.
- Basuny AM, Arafat SM, Farag HA, Soliman HM (2016) Production of mixture more resistance from some vegetable oils for frying process. International Journal of Chemical and Natural Science 4: 365-373.
- Nallusamy S (2006) The role of palm oil in the snack food industry, Presented at International Palm Oil Trade Fair and Seminar, Kuala Lumpur, Malysia.
- Abdluakerim SM, Myat MW, Ghazali MH (2010) Sensory and physicochemical qualities of palm olein and sesame seed oil blends during frying of banana chips. Journal of Agricultural Science 2(4): 18- 29.
- Naghshineh M, Mirhosseini H (2010) Effect of frying condition on physicochemical properties of palm olein-olive oil blends Journal Food Agricultural and Environmental 8(3&4): 175-178.
- Al-Khusaibi MK, Gordan MH, Lovegrove JA, Niranjan K (2012) Frying of potato chips in a blend of canola oil and palm olein: Changes in levels of individual fatty acids and tocols. International Journal of Food Science and Technology 47(8): 1701-1709.
- Chen JF, Tai CY, Chen YC, Chen BH (2000) Effects of conjugated linoleic acid on the oxidation stability of model lipids during heating and illumination. Food Chemistry 72(2): 199-206.
- Yoon SH, Kim SK, Kim KH, Kwon TW, et al. (1987) Evaluation of physicochemical changes in cooking oil during heating. Journal of American Oil Chemists Society 64(6): 870-873.
- Gunstone FD (2006) Methods of analysis to determined the quality of oils. In Warner, K. (Eds). Modifying Lipids for Use in Food. Boca Ratan: CRC Press p. 115-117.
- Gunstone FD (2004) The chemistry of oils and fats: sources, composition, properties and uses. CRC Press Blackwell Publishing Ltd.
- Arafat SM, Basuny AM, Abd-Hady MM (2013) Production of low acidity rice bran oil by heating process. Peak Journal of Food Science and Technology 1(2): 13-18.
- AOAC (2005) Official methods of Association of Agricultural Chemicals. (18th edn). Washington, DC: AOAC, USA.
- Nielson SS (1998) Food analysis. (2nd edn). Gaithersburg, Maryland: Aspen Inc. pp. 222-223.
- Wu PF, Nawar WW (1986) A technique for monitoring the quality of used frying oils. Journal of American Oil Chemists Society 63(10): 1363-1367.
- Waltking AE, Wessels H (1981) Chromatographic separation of polar and non-polar components of frying oils. Journal Association of Official Analytical Chemistry 64(): 1329-1330.
- Firestone D (2009) Official method and recommended practices of the AOCS. (6th edn). American Oil Chemists Society Press Chanpaign II.
- Seetharamaiah GS, Prabakar JV (1986) Oryzanol content of Indian rice bran oil and its extraction from soap stock. Journal Food Science and Technology 23: 270-275.
- Farhoosh R (2007) The effect of operational parameters of the Rancimat method on the determination of the oxidation stability measures and shelf-life prediction of soybean oil. Journal of the American Oil Chemists Society 84(3): 205-209.
- Mathaus B (2006) Utilization of high-oleic rapeseed oil for deep-fat frying of fresh fries compared to other commonly used edible oils. Eur J Lipid Sci Technol 108: 200-211.
- Al-Khusaibi MK, Niranjian K (2012) The impact of blanching and high-pressure pretreatments on oil uptake of fried potato slices. Food Bioprocess Technol 5(6): 2392-2400.
- Shahidi F, Wanasundara UN (2002) Method for measuring oxidative rancidity in fats and oils. In: Akoh CC, Min DB (Eds.), Food Lipids: Chemistry, Nutrition and Biotechnology (2nd edn.), New York: Marcel Dekker. Inc. pp. 465-482.
- Mohamed-Sulieman SEA, El-Makhzangi, Rmadan MF (2006) Antiradical performance and physico-chemical characteristics of vegetable oil upon frying frensh fries: a preliminary comparative study. J Food Lipids 13(3):259-276.
- Dobarganes, M. C.; Velasco, J. andDieffenbacher, A. (2003) Determination of polar compounds, polymerized and oxidized triacylglycerols, and diacylglycerols in oils and fats: results of collaborative studies and the standardized method. Pure Appl Chem 72(8): 1563-1575.
- Gertz C, Kochhar SP (2001) A new method to determine oxidative stability of vegetable fats and oils at simulated frying temperature. Ol Crops Gras Lipids 8(1): 82-88.
- Maskan M, Bagic H (2003) The recovery of used sunflower seed oil utilized in repeated deep-fat frying process. Eur. Food Res. Technol. 218(1): 26-31.
- Shin TS, Godber JS, Martin DE, Wells JH (1997) Hydrolytic stability and changes in e vitamers and oryzanol of extruded rice bran during storage. J Food Sci 62(4): 704-728.
- Sanchez-Muniz FJ, Botega D, diLorenzo L, Marmesat SL, Bastida L, et al. (2007) A non-extractable condensed-tannins fiber reduces thermal oxidation in oils at frying temperature. Eur J Food Sci Technol 109(2): 1218-1225.






























