In vitro Determination of Antioxidant Activities of the Fractions Obtained from Adansonia Digitata L. (baobab) Stem Bark Ethanolic Extract using Different Parameters
Ojochenemi E Yakubu1, Okwesili Fred C Nwodo2, Christopher Shaibu1, Silas V Tatah1, Moses A Abah1 and Sunday Gabriel1
1Department of Biochemistry, Federal University Wukari, Nigeria
2Department of Biochemistry, University of Nigeria, Nsukka, Nigeria
Submission: December 12, 2018; Published: January 18, 2019
*Corresponding author: Ojochenemi Ejeh Yakubu, Pharmacological Biochemistry/Toxicology Research Unit, Department of Biochemistry, Federal University Wukari, Nigeria
How to cite this article: Ojochenemi E Y, Okwesili F C N, Silas V T, Moses A ,Sunday G. In vitro Determination of Antioxidant Activities of the Fractions Obtained from Adansonia Digitata l. (baobab) Stem Bark Ethanolic Extract using Different Parameters. Curr Trends Biomedical Eng & Biosci. 2019; 17(5): 555973. DOI: 10.19080/CTBEB.2019.17.555973
Abstract
Medicinal plants have been utilized to treat acute and chronic disorders for many years. The basis for such utilization however is based on the potential use of phytochemicals to manage a plethora of chronic diseases including cancer, inflammatory diseases and cardiovascular abnormalities. In this study, the in vitro antioxidant capacity (TAC), total phenolic content (TPC) and total flavonoids content (TFC) β-carotene bleaching inhibition assay and metal chelating activity of the fractions obtained from ethanolic extract of the stem bark of Adansonia digitata were determined using Spectrophotometric methods. Extraction was carried out using absolute ethanol and fractionation was carried out using organic solvents of different polarities, beginning from n-Hexane, ethyl-acetate, ethanol, methanol and finally distilled water. Eighteen fractions were obtained from the ethanolic extract using the solvents. Folin Ciocalteau is a mixture of phosphomolybdate and phosphotungstate which was used for the Spectrophotometric in vitro assay of the Total Phenolic Content (TPC). Total Flavonoid Contents (TFC) was determined by the aluminium colorimetric method, with quercetin as reference standard. β-Carotene bleaching inhibition assay, the metal chelating activity of the extract fractions with ferrous ions were also carried out. The results obtained from the study revealed that fractions 6 and 7 (2900.00 mg/ml TE and 2410.46 mg/ml TE) possessed the highest 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity while fraction 8 and 9 (355.56 mg/ml TE and 420.26 mg/ml TE) possessed the lowest activities. Furthermore, Fractions 10 and 13 (1415.23 mg/ml GAE and 664.76 mg/ml GAE) showed the highest total phenolic content (TPC) while fraction 16 and 9 (2.86 mg/ml GAE and 4.29 mg/ml GAE) had the lowest values respectively. Fractions 5 and 9 showed the highest Total Flavonoids Content (TFC) followed by fraction 12, and the lowest was observed in fractions 2 and18. The highest concentration for β-Carotene Bleaching Inhibition Assay was observed in fractions 12 and 9, and the lowest β-Carotene concentration was observed in fractions 7 and 8. Metal Chelating Inhibition Activity revealed that fractions 12 and 5 have the highest concentration, 7 and 8 with the lowest concentration. In conclusion, the free radicals scavenging potentials of this plant extract probably contribute to the effectiveness of the plant as a medicinal plant. The secondary metabolites in the plant have been found to be of medicinal importance both in preventive and curative medicine, especially as revealed by fractions 5, 6, 7, 10, 11 and 12, the peak of which were found in fractions 5 and 12.
Keywords: Antioxidant; Fractions: Adansonia digitata; Different; Ethanolic, Parameters
Abbrevations: TAC: Antioxidant Capacity; TPC: Total Phenolic Content; TFC: Total Flavonoids Content; DPPH: 1-Diphenyl-2-Picrylhydrazyl; ROS: Reactive Oxygen Species;
Introduction
The oxygen consumption inherent in cells growth leads to the generation of series of oxygen free radicals. Highly active free radicals and their uncontrolled production are responsible for numerous pathological processes such as cell tumor and coronary heart diseases [1-3]. Oxidative stress plays a major role in the development of chronic and degenerative diseases such as cancer, arthritis, aging, autoimmune disorders, cardiovascular and neurodegenerative diseases. Generation of highly reactive oxygen species (ROS) is an integral feature of normal cellular function [4]. Damages due to free radicals caused by ROS leads to several damaging effects as they can attack lipids, protein/enzymes, carbohydrates, and DNA in cells and tissues.
They induce undesirable oxidation, causing membrane damage, protein modification, DNA damage, and cell death induced by DNA fragmentation and lipid peroxidation [4]. Antioxidants have been known to play protective role in human body against deleterious effects of reactive free radicals. They can significantly delay or prevent the oxidation of easily oxidizable substances [5,6]. Natural antioxidants are classified according to their mechanism of action as chain-breaking antioxidants which scavenge free radicals or inhibit the initiation step or interrupt the propagation step of oxidation of lipid and as preventive antioxidants which slow the rate of oxidation by several actions but do not convert free radicals [7-11].
that also act as antioxidants by many potential pathways such as free radical-scavenging, oxygen radical absorbance, and chelating of metal ions [12]. They are naturally present in fruits and vegetables and also used as supplements or medicines. Research has shown that fruits and vegetables contain other antioxidant nutrients, in addition to vitamins C and E, and carotenoids, which significantly contribute to their total antioxidant capacity [13,14]. The major part of those antioxidant nutrients is polyphenolic compounds, which are components of fruits and vegetables having strong antioxidant capacity [13,14].
Flavonoids have a wide range of biological activities, such as cell-proliferation-inhibiting, apoptosis-inducing, enzymeinhibiting, antibacterial, and antioxidant effects. Some findings indicate that flavonoids possess various clinical properties, such as anti-atherosclerotic, anti-inflammatory, anti-tumor, antithrombogenic, anti-osteoporotic, and antiviral effects [15,16]. Interest in traditional medicines has grown in recent years as they are typically low in toxicity, rarely produce complications and have beneficial pharmacological activities [7]. Epidemiological and in vitro studies on medicinal plants and vegetables strongly have supported the idea that plant constituents with antioxidant activity are capable of exerting protective effects against oxidative stress in biological systems [13].
Adansonia digitata, commonly called baobab is most widespread of the Adansonia species on the African continent, and is majorly found in the hot, dry savannahs of sub-Saharan Africa. Different parts of Adansonia digitata solvent extracts have been reported to possess medicinal properties and are currently been used in treatment of various ailments such as diarrhea, fever induced malaria, inflammation, scurvy, cough, dysentery, small pox, measles, kidney and bladder diseases, blood clearing and asthma (Van and Gericke, 2000). This study examined the flavonoid, total phenolic content, and antioxidant potentials of aqueous and ethanolic extracts of Adansonia digitata stem bark. This study is aimed at determining the in vitro antioxidant activities of the fractions obtained from Adansonia digitata (baobab) stem bark; ethanolic extract using different parameters.
Materials and Methods
Sample collection
The healthy stem bark of the plant Adansonia digitata were collected from Biological garden within Federal University Wukari campus, Wukari, Taraba State, Nigeria.
Sample preparation
The leaves and stem bark were examined to be free from disease. Only healthy plant parts were used. The stem bark was cut into pieces using kitchen knife and were dried under shade for one week to reduce moisture content and to prevent enzyme action. The dried leaves and stem bark was pulverized using wooden mortar and pestle into powdered form.
Ethanolic extraction
The pulverized sample was soaked in sufficient volume of ethanol for 48 hours at room temperature. It was continually stirred after each 8 hours. After 48 hours, the extract was filtered out first using a clean white sieving mesh and then using Whatman No. 1 filter paper. The concentrated extract was then transferred to air-tight containers, corked and preserved in the refrigerator at 4oC until required.
Fractionation of ethanolic extract
The ethanol extract was subjected to column chromatograph to separate the extract into its component fractions. Silica gel was used in packing the column while varying solvent combinations of increasing polarity were used as the mobile phase.
Packing of column
This was done according the method of Yakubu et al. [18]. The lower part of the glass column was stocked with glass wool with the aid of glass rod. 235g of silica gel (100-200 mesh size) was dissolved in 255ml of absolute n-Hexane to make the slurry. The chromatographic column (30mm diameter by 40cmheight) was packed with silica gel and was allowed free flow of the solvent into a conical flask below. The set up was seen to be in order when the solvent drained freely without carrying either the silica gel or glass wool into the tap. At the end of the packing process, the tap was locked, and the column was allowed 24 hours to stabilize after which, the clear solvent at the top of the silica gel was allowed to drain down the silica gel meniscus.
Elution
The method of Yakubu et al. [18] was adopted for the elution. The extract (2g) was dissolved in 15ml absolute ethanol and the solution was applied unto a chromatographic column (30mm diameter by 400mm height). Elution of the extract was done with solvent system of gradually increasing polarity, beginning from n-Hexane, ethyl acetate, ethanol, methanol and finally water. The following ratios of solvent combinations were sequentially used in the elution process:
i. n-Hexane : ethyl acetate 100:0, 50:50;
ii. Ethyl acetate : ethanol 100:0, 50:50;
iii. Ethanol : methanol 100:0, 50:50;
iv. Methanol : water 100:0, 50:50
v. Water :100
A measured volume (200ml) of each solvent combination was poured into the column each time using Separatory funnel, The eluted fractions were collected in aliquots of 100ml using beakers.
Determination of total antioxidant capacity (TAC)
DPPH radical scavenging activity was measured as per the procedure of (Shimada et al., 1992). The absorbance was measured in triplicate for each fraction. Total antioxidant capacity (TAC) was calculated as mg/ml Trolox equivalent (TE) using the regression equation from calibration curve.
Determination of total flavonoid content
Flavonoids were determined using the aluminium chloride colorimetric method of Chang et al. [19]. Quercetin standard was used for derivation of the calibration curve. Total flavonoids were expressed as mg/ml quercetin equivalent (QE).
Determination of total phenolic content
Total phenolic content (TPC) of the extract was estimated following the phosphomolybdic/ phosphotungstic acid complex procedure of [21].
β-Carotene bleaching inhibition assay
In this assay, antioxidant activity was determined by measuring the inhibition conjugated dienehydroperoxides arising from linoleic acid oxidation [22].
Metal chelating activity
The metal chelating activity of the extract fractions with ferrous ions were measured in triplicates following the method [23]. The chelating activity of the extract at different concentrations was calculated as:
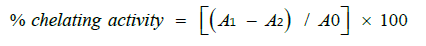
Where A0, Absorbance of the control (without extract); A₁, Absorbance of reaction mixture and A₂, Absorbance without FeCl₂.
Results
The results obtained from the study revealed that fractions 6 and 7 possessed the highest 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity and closely followed by fraction 5, while fraction 8 and 9 possessed the lowest activities. Fractions 14 and 16 possessed almost the same antioxidant activities (Figure 1).
Fraction 1=n-Hexane (100:00), 2= n-Hexane (100:00), 3= n-Hexane/ethyl acetate (100:00), 4= n-Hexane/ethyl acetate (50:50) 5=Ethyl acetate/ethanol (100:00), 6= Ethyl-acetate/ ethanol (100:00), 7= Ethyl-acetate/ethanol (50:50), 8=Ethyl acetate/ethanol (50:50), 9= Ethanol/ methanol (100:00), 10= Ethanol/ methanol (100:00), 11= Ethanol/Methanol (50:50), 12= Ethanol/Methanol (50:50), 13= Methanol /water (100:00), 14= Methanol /water (100:00), 15= Methanol /water (50:50), 16= Methanol /water (50:50), 17=Water (100:00), 18= Water (100:00)
The results obtained from the study showed that Fractions 10 and 13 have the highest total phenolic content (TPC) while fractions 16 and 9 were recorded the lowest values. Fractions 2, 4, and 7 have the same amount of TPC (Figure 2).

Fraction 1=n-Hexane (100:00), 2= n-Hexane (100:00), 3= n-Hexane/ethyl acetate (100:00), 4= n-Hexane/ethyl acetate (50:50) 5=Ethyl acetate/ethanol (100:00), 6= Ethyl-acetate/ ethanol (100:00), 7= Ethyl-acetate/ethanol (50:50), 8=Ethyl acetate/ethanol (50:50), 9= Ethanol/ methanol (100:00), 10= Ethanol/ methanol (100:00), 11= Ethanol/Methanol (50:50), 12= Ethanol/Methanol (50:50), 13= Methanol /water (100:00), 14= Methanol /water (100:00), 15= Methanol /water (50:50), 16= Methanol /water (50:50), 17=Water (100:00), 18= Water(100:00) The results obtained from the study showed that Fractions 5 and 9 showed the highest Total Flavonoids Content (TFC) followed by fraction 12, and the lowest was observed in fractions 2 and 18 (Figure 3).

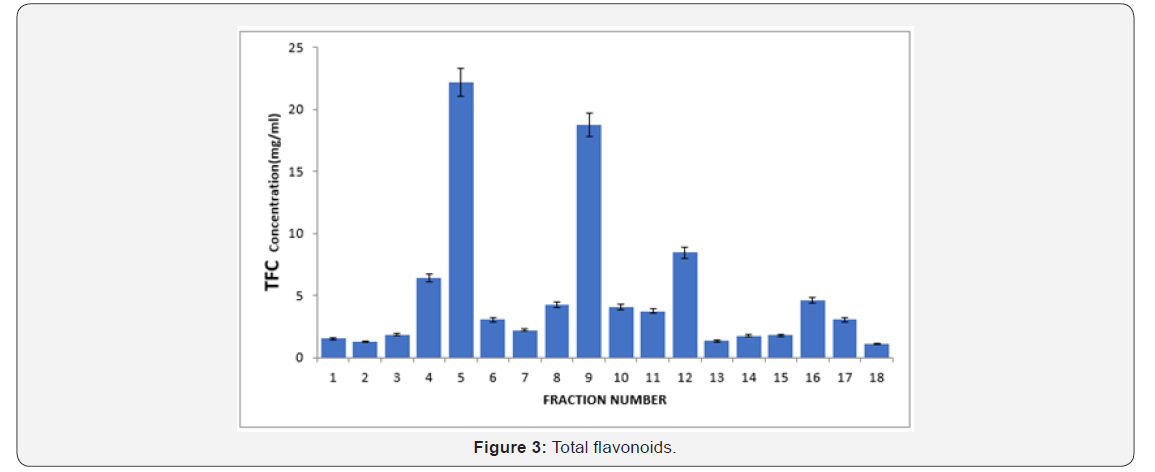
Fraction 1=n-Hexane (100:00), 2= n-Hexane (100:00), 3= n-Hexane/ethyl acetate (100:00), 4= n-Hexane/ethyl acetate (50:50) 5=Ethyl acetate/ethanol (100:00), 6= Ethyl-acetate/ ethanol (100:00), 7= Ethyl-acetate/ethanol (50:50), 8=Ethyl acetate/ethanol (50:50), 9= Ethanol/ methanol (100:00), 10= Ethanol/ methanol (100:00), 11= Ethanol/Methanol (50:50), 12= Ethanol/Methanol (50:50), 13= Methanol /water (100:00), 14= Methanol /water (100:00), 15= Methanol /water (50:50), 16= Methanol /water (50:50), 17=Water (100:00), 18= Water(100:00)

The results obtained from Metal Chelating Inhibition Assay (Figure 4), revealed that fractions 12 and 5 have the highest concentration, with the lowest observed in fractions 7 and 8.
Fraction 1=n-Hexane (100:00), 2= n-Hexane (100:00), 3= n-Hexane/ethyl acetate (100:00), 4= n-Hexane/ethyl acetate (50:50) 5=Ethyl acetate/ethanol (100:00), 6= Ethyl-acetate/ ethanol (100:00), 7= Ethyl-acetate/ethanol (50:50), 8=Ethyl acetate/ethanol (50:50), 9= Ethanol/ methanol (100:00), 10= Ethanol/ methanol (100:00), 11= Ethanol/Methanol (50:50), 12= Ethanol/Methanol (50:50), 13= Methanol /water (100:00), 14= Methanol /water (100:00), 15= Methanol /water (50:50), 16= Methanol /water (50:50), 17=Water (100:00), 18= Water(100:00)
Figure 5 shows the result for β-Carotene Bleaching Inhibition Assay. The highest concentration for β-Carotene Bleaching Inhibition Assay was observed in fractions 12 and 9 followed by fraction 10, and the lowestβ-Carotene concentration was observed in fraction 7 and 8.
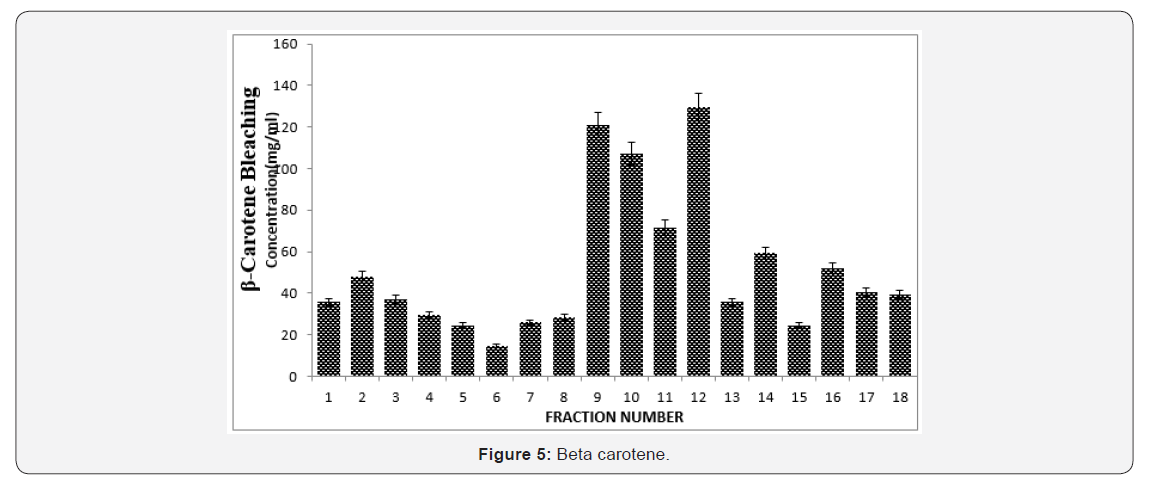
Fraction 1=n-Hexane (100:00), 2= n-Hexane (100:00), 3= n-Hexane/ethyl acetate (100:00), 4= n-Hexane/ethyl acetate (50:50) 5=Ethyl acetate/ethanol (100:00), 6= Ethyl-acetate/ ethanol (100:00), 7= Ethyl-acetate/ethanol (50:50), 8=Ethyl acetate/ethanol (50:50), 9= Ethanol/ methanol (100:00), 10= Ethanol/ methanol (100:00), 11= Ethanol/Methanol (50:50), 12= Ethanol/Methanol (50:50), 13= Methanol /water (100:00), 14= Methanol /water (100:00), 15= Methanol /water (50:50), 16= Methanol /water (50:50), 17=Water (100:00), 18= Water(100:00)
Discussion
Total antioxidant capacity
The Total Antioxidant Capacity of the ethanolic extract of the stem bark of Adansonia digitata ranges from 290.000 - 355.556 mg/mlTE . It showed that it possessed an appreciable amount of antioxidant activity. This is in consonance with some studies that medicinal plants used in traditional medicine and healing are one of the sources of antioxidants [24]. The antioxidants activities of the stem bark investigated with DPPH free radical scavenging assay showed valuable results which contributed to the interest of screening the plants extract for further medical related indices and to evaluate its therapeutic values. DPPH, a stable Free radical with characteristics absorption at 517nm, was used to study the radical scavenging effects of the extract. Evidence has it that DPPH Free radical scavenging by antioxidants is due to their hydrogen donating ability [25].
Free radical and other reactive oxygen species (ROS), such as superoxide anions, hydroxyl radicals, and hydrogen peroxide are an entire class of highly reactive species derived from the normal metabolism of oxygen or from exogenous factors and agents [26]. Oxidative damage to crucial cellular molecules, induced by ROS, has been implicated as a possible factor in the etiology of several human diseases, including cancer, cardiovascular diseases and aging [27]. As antioxidants donate protons to these radicals, the absorption decreases. The decrease in the absorption is taken as a measure of the radical scavenging. Free radical scavenging capacities of the extract, measured by DPPH assay, are show in figure 1. The antioxidant properties determined show that the solvents used were able extract substances with antioxidant potency, especially the solvent combinations for fractions 5, 6 and 7 (ethyl acetate and ethanol fractions).
Phenolic and flavonoid contents
Different phytochemicals have various protective and therapeutic effects which are essential to prevent diseases and maintain a state of wellbeing. Fractions of ethanolic extract of the stem bark of Adansonia digitata were analyzed for its phytoconstituents. The quantitative estimation of the phytochemical constituents of Adansonia digitata showed that the medicinal plant is rich in total phenols, total flavonoids according to the data shown in the Figure 2, The low phenolic contents of fractions 5 -7 in relation to their antioxidant activity and flavonoids content suggests that the extract may have many phenolic contents but flavonoids are basically responsible for its antioxidant activities as shown in figure 3, it is well that plant flavonoids and phenols in general, are highly effective free radical scavenging and antioxidants. Polyphenol and flavonoids are used for the prevention and cure of various diseases which are mainly associated with free radicals [16,28]. The phenolic compounds have been recognized as antioxidant agents, which act as free radical oxidation terminators and have been known to show medicinal activity as well as for exhibiting physiological functions [29,30]. It has been reported that compounds such as the flavonoids, which contain hydroxyls, are responsible for the radical scavenging effects of most plants [31]. The mechanisms of action of the flavonoids are through scavenging or chelating processes [32]. It is well established that plant phenolics, in general are highly effective in free radical scavenging and they are antioxidants. Several investigations have shown that many of these plants have antioxidant activities that could be therapeutically beneficial, and it has been mentioned that the antioxidant potential of plants might be due to their phenolic components [33].
The presence of these phytochemicals in fractions of plants extract is thus a significant finding of the present study. It is well known that oxidative stress included by oxygen-free radicals and resultant tissue damage is the hallmarks of several chronic disorders and cell death [34]. The therapeutic potentials of medicinal plants as natural antioxidants in reducing such free radical induced tissue damage [35] and in the maintenance of health and protection from some age-related degenerative disorders such as cancer and coronary heart diseases is established [9].
Metal chelating activity
The chelating of Fe2+ by extracts was estimated by the method of Dinis et al. [23]. Ferrozine can quantitatively form complexes with Fe2+. However, in the presence of chelating agents, the complex formation is disrupted with the result that the red colour of the complex is decreased. Measurement of colour reduction, therefore, allows the estimation of the chelating activity of the coexisting chelator. The transition metal ion, Fe2+ possess the ability to move single electrons by virtue of which it can allow the formation and propagation of many radical reactions, even starting with relatively non-reactive radicals [36]. The main strategy to avoid ROS generation that is associated with redox active metal catalysis involves chelating of the metal ions. Fractions 5 and 12 (figure 4) are the most active extract interfered with the formation of ferrous and ferrozine complex, suggesting that it has chelating activity and captures ferrous ion before ferrozine.
Beta-Carotene Bleaching (BCB) inhibition Assay
Figure 4 showed the results of the extracts as measured by the bleaching of β-carotene. The addition of the extracts prevented the bleaching of b-carotene to different degrees. β-Carotene in this model system undergoes rapid discoloration in the absence of an antioxidant (control). This is because of the coupled oxidation of b-carotene and linoleic acid, which generates free radicals. The linoleic acid free radicals, formed upon the abstraction of a hydrogen atom from one of its diallylic methylene groups, attacks the highly unsaturated β-carotene molecules. As a result, β-carotene will be oxidized and broken down in part; subsequently, the system loses its chromophore and characteristic orange color, which was monitored spectrophotometrically [37-40]. The presence of different antioxidants (plant extract/fractions) can hinder the extent of β-carotene bleaching by neutralizing the linoleate-free radical and other free radicals formed in the system. From figure 5, it was crystal clear that different fractions demonstrated different potencies for the inhibition of this bleaching agent, with fractions 9 and 12 performing better than other fractions of the extract.
Conclusion
In conclusion, the present investigation of all the different methods of determining antioxidant activity shows potential antioxidant and free radical scavenging activity. The high antioxidant activity exhibited by Adansonia digitata stem bark extracts provided justification for the therapeutic use of this plant in folk medicine due to the phytochemical constituents. The results therefore suggest that the extract (especially fractions 5, 6, 7, 11and 12) could be potential sources of natural antioxidant that could be of great importance for the treatment of free radical related diseases because of their consistency in all the parameters studied.
References
- Karadenz F, Burdurlu HS, Koca N (2005) Antioxidant activity of selected fruits and vegetables grown in turkey. Turk J Agric 29: 297-303.
- Barros L, Joao FM, Queiros B, Ferreira IC, Baptista P (2007) Total phenol, ascorbic acid, β-carotene and lycopene in Portuguese wild edible mushroom and their antioxidant activities. Food Chemistry 103(2): 413-419.
- Jagadish LK, Krishnan VV, Shenbhagaraman R, Kaviyarasan V (2009) Comparative study on the antioxidant, anticancer and antimicrobial property of Agaricus bisporus imbach before and after boiling. Afr J Biotechnol 8(4): 654-661.
- Ravindra PS, Shashwat S, Suman K (2004) Free Radicals and Oxidative Stress in Neurodegenerative Diseases: Relevance of Dietary Antioxidants. JIACM 5(3): 218-225.
- Atrooz OM (2009) The antioxidant activity and polyphenolic contents of different plant seeds extracts. Pak J Biol Sci 12(15): 1063-1068.
- Kim JH, Kim SJ, Park HR, Choi J, Cheoul JY, et al. (2009) The different antioxidant and anticancer activities depending on the colour of oyster mushroom. J Med Plants Res 3(12):1016-1020.
- Ou B, Huang D, Hampsch-Woodill M, Flanagan JA, Deemer EK (2002) Analysis of antioxidant activities of common vegetables employing oxygen radical absorbance capacity (ORAC) and Ferric reducing antioxidant power (FRAP) assays: A comparative study. J Agric Food Chem 50(11): 3122-3128.
- Thaipong K, Boonprakob U, Crosby K, Cisneros-Zevallos L, Hawkins BD (2006) Comparison of ABTS, DPPH, FRAP and ORAC assays for estimating antioxidant activity from guava fruit extracts. J Food Comp Anal 19(6-7): 669-675.
- Othman A, Ismail A, Abdul Ghani N, Adenan I (2007) Antioxidant capacity and phenolic cocoa beans. Food Chemistry 100(4): 1523-1530.
- Hodzic Z, Pasalic H, Memiseric A, Srabovic M, Saletovic M (2008) The influence of total phenolic content on antioxidant capacity in the whole grain extracts. Euro J Sci Res 28: 471-477.
- Semalty M, Semalty A, Joshi GP, Rawat MSM (2009) Comparison of in vitro antioxidant activity of Trigonella foenum-graecum and T. corniculata seed. Res J Phytochem 3(3): 63-67.
- Halliwell B, Aeschbach R, Loliger J, Aruoma OI (1995) The characterization of antioxidants. Food Chem Toxicol 33(7): 601–617.
- Cao G, Sofic E, Prior RL (1996) Antioxidant capacity of tea and common vegetables. Journal of Agricultural and Food Chemistry 44(11): 3426–3431.
- Wang H, Cao G, Prior RL (1996) Total antioxidant capacity of fruits. Journal of Agricultural and Food Chemistry 44(3): 701-705.
- Cook NC, Samman S (1996) Flavonoids-chemistry, metabolism, cardioprotective effects, and dietary resources. The Journal of Nutritional Biochemistry 7(2): 66-76.
- Havesteen B (1983) The biochemistry and medical significance of the flavonoids. Pharmacol Ther 68(48): 1141-1148.
- Endo J, Nakamura Y (1993) Reconsideration of the concepts of the large intestine and the small intestine in Chinese traditional medicine. Journal of Japanese History of Medicine 39(2): 157-168.
- Yakubu OE, Nwodo OFC, Joshua PE, Ugwu MN, Odu, AD, et al. (2014) Fractionation and determination of total antioxidant capacity, total phenolic and total flavonoids contents of aqueous, ethanol and n-hexane extracts of Vitex doniana leaves. African J Biotech 13(5): 693-698.
- Chang CC, Yang MH, Wen HM Chern JC (2002) Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal 10: 178-182.
- Brady O (2011) The characterization and bioactivity determination of Adansonia digitata L. fruit pulp, for commercial product development. Thesis of Bachelor of Science in Nutraceuticals for Health and Nutrition Dublin Institute of Technology, Cathal Brugha Street, 117 p.
- Velioglu YS, Mazza G, Gao L, Oomah BD (1998) Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J Agric Food Chem 46(10): 4113-4117.
- Jayaprakasha GK, Mandadi KK, Poulose SM, Jadegoud Y, Nagana-Gowda GA, et al. (2001) Inhibition of colon cancer cell growth and antioxidant activity of bioactive compounds from Poncirus trifoliata (L.) Med Chem 15(14): 4923-4932.
- Dinis TCP, Madeira VMC, Almeida MLM (1994) Action of phenolicderivates (acetoaminophen, salycilate and 5-aminosalycilate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys 315(1): 161-169.
- Jin D, Russell J M (2010) Plant Phenolics: Extraction, Analysis and their Antioxidant and Anticancer Properties. Molecules 15(10): 7313-7352.
- Iwueke AV Nwodo OFC (2008) Anthihyperglycaemic effect of aqueous extract of Daniella oliveri and Sarcocephalus latifolius roots on key carbohydrate metabolic enzymes and glycogen in experimental diabetes. Biokemistri 10(2): 63-70.
- Valko M, Leibfritz D, Moncola J (2007) Free radicals and antioxidants in normal physiological functions and human disease Review. Int J Biochem Cell Biol 39(1): 44-84.
- Faujam H, Noriham A, Norrakiah AS, Babji AS (2009) Antioxidant activity of plants methanolic extracts containing phenolic compound. Afr J Biotechnol 8(3): 484-489.
- George F, Zohar K, Harinder PS, Makkar KB (2002) The biological action of saponins in animal systems: a review. Br J Nutr 88(6): 587-605.
- Shahidi F, Wanasundara JPD (1992) Total antioxidant capacity of fruits. Crit. Rev. Food Sc. Nutr. 32(2):45-46
- Sofowora A (1993) Medicinal plants and traditional medicine in Africa, Spectrum books, Ind, Ibadan, Nigeria. p. 289.
- Langenheim JH (1994) Higher plant terpenoids: A phytocentric overview of theirecological roles. J Chem Ecol 20(6): 1223-1280.
- Morrissey JP, Osbourn AE (1999) Fungal resistance to plant antibiotics as a mechanism of pathogenesis. Microbiol Mol Biol Rev 63(3): 708-724.
- Mandal SM, Chakraborty D, Dey S (2010) Phenolic acids act as signaling molecules in plant–microbe symbioses. Plant Signal Behav 5(4): 359-368.
- Mangan JL (1988) Nutritional effects of tannins in animal feeds. Nutr Res Rev 1(1): 209-231.Wang H, Cao G, Prior RL (1997) Oxygen radical absorbing capacity of anthocyanins. Journal of Agricultural and Food Chemistry 45(2): 304-309.
- Bouayed J, Hoffmann L, Bohn T (2011) Total phenolics, flavonoids, anthocyanins and antioxidant activity following simulated gastro-intestinal contents. Food Chem 128(1): 14-21.
- Aboul-Enein AM, El Baz FK, El-Baroty GS, Youssef AM, Abd El-Baky HH (2003) Antioxidant activity of algal extracts on lipid peroxidation. J Med Sci 3(1): 87-98.
- Moorachian ME (2000) Phytochemicals: Why and How? Tastings, 4-5. Costa MA, Zia ZQ, Davin LB and Lewis, N.G. (1999). Chapter Four: Toward Engineering the Metabolic Pathways of Cancer-Preventing Lignans in Cereal Grains and Other Crops. In Recent Advances in Phytochemistry, vol. 33, Phytochemicals in Human Health Protection, Nutrition, and Plant Defense ed. JT Romeo, New York, 67-87.
- Narasinga R (2003) Bioactive phytochemicals in Indian foods and their potential in health promotion and disease prevention. Asia Pac J Clin Nutr 12(1): 9-22.
- Yakubu OE, Imarenezor EPK, Udeh SMC (2016) Total antioxidant capacity, phenolic and flavonoids contents of partially purified aqueous extract of Vitex doniana leaves. FUW Trends in Science and Technology Journal 1(1): 211-214.
- Yakubu OE (2017) Amelioration of some indices of diabetic complications using Senna occidentalis leaf extract in alloxan-induced diabetes in rats. AASCIT Journal of Biosciences 3(4): 24-28.






























