Hypoglycaemic Effect of Leaves and Stem Bark of Adansonia Digitata (L) (Baobab) Ethanolic Extract in Alloxan-Induced Diabetes in Male Wistar Rats
Ojochenemi Ejeh Yakubu1, Chinedu Imo1, Christopher Shaibu1, John Akighir2 and Daniel Simon Ameh1
1Department of Biochemistry, Federal University Wukari, Nigeria
2Department of Biochemistry, University of Agriculture, Nigeria
Submission: November 27, 2018; Published: January 17, 2019
*Corresponding author: Ojochenemi Ejeh Yakubu, Pharmacological Biochemistry/Toxicology Research Unit, Department of Biochemistry, Federal University Wukari, Nigeria
How to cite this article: Ojochenemi E Y, Chinedu I, Christopher S, John A, Daniel S A. Hypoglycaemic Effect of Leaves and Stem Bark of Adansonia Digitata (L) (Baobab) Ethanolic Extract in Alloxan-Induced Diabetes in Male Wistar Rats. Curr Trends Biomedical Eng & Biosci. 2019; 17(5): 555972. DOI: 10.19080/CTBEB.2019.17.555972
Abstract
This study was carried out to evaluate the effect of the ethanolic leaf extract of Adansonia digitata on blood glucose and some biochemical parameters in alloxan-induced diabetic Wistar rats. Twenty-five Wistar rats were distributed into five groups of five animals each. Rats were administered alloxan (150 mg/kg) intraperitoneally and were monitored for 72hr for the development of hyperglycemia. Group 1 served as normal control, group 2, diabetic control, while Groups 3, 4 and were diabetic rats treated orally with ethanolic extract of Adansonia digitata (100mg/kg) daily for 21-days, while Group 5 were diabetic rats treated with anti-diabetic drug (glibenclamide). The blood glucose level and body weight were determined weekly for 21-days using Accu-Check Active glucometer and weighing balance. The result of the study indicated a significantly reduced blood glucose level and a significantly (p<0.05) reduced liver function parameters in alloxan-induced diabetic treated rats compared with normal control and diabetic control. However, there was no specific pattern of increase/decrease in the full blood count parameters. The presence of flavonoids, tannins, saponins, terpenoids and steroids could be responsible for the effects elicited by the extract.
Keywords: Glibenclamide; Blood glucose; Glucometer; Phytochemicals; Alkaloids; Flavonoids
Abbrevations: ALT: Alanine Aminotransferase; AST: Aspartate Aminotransferase; ALP: Alkaline Phosphatase; BIL: Bilirubin; K: Potassium; RBC: Red Blood Cell Count; HGB: Haemoglobin; WBC: White Blood Cell; IDDM: Insulin Dependent Diabetes Mellitus; SVCT 1: Sodium-Dependent Vitamin C Transporter 1; Glut 2: Glucose Transporter Isoform 2
Introduction
Medicinal plants contain biologically active chemical substances (phytochemicals) such as saponins, tannins, essential oils, flavonoids, alkaloids and other chemical compounds, which have preventive and curative properties. These complex chemical substances of different compositions are found as secondary plant metabolites in one or more of these plants and are useful for humanity [1]. In view of many diseases defiling drugs, health practices are now changing from curative to preventive medicine. Phytochemicals popular in preventive medicine are flavonoids, polyphenols, saponins, lignoids and vitamins. Also, a knowledge of the chemical constituents of plants is desirable, not only for the discovery of therapeutic agents, but also because such information may be of value in disclosing new sources of such economic materials as tannins, oils, gums, which are precursors for the synthesis of complex chemical substances, etc. In addition, the knowledge of the chemical constituents of plants would further be valuable in discovering the actual value of folkloric remedies [2].
Adansonia digitata L. called the baobab tree in both English and French is very characteristic of the sahelian region and belongs to the Malvaceae family [3]. The plant is a massive tree with a very large trunk (up to 10m diameter) which can grow up 25m in height and may live for hundreds of years. The plant is widespread throughout the hot and drier regions of tropical Africa [4]. Baobab tree has multi-purpose and every part of the plant is reported to be useful [4,5]. The leaves, for instance are used in preparation of soup, seeds are used as a thickening agent in soups, but they can be fermented and used as flavoring agent or roasted and eaten as snacks [6]. The pulp is either sucked or made into a drink while the bark is used in making ropes [4,7]. The different part of the plant provides food, shelter, clothing and medicine as well as material for hunting and fishing [8-10]. Baobab tree provides income and employment to rural and urban households.
Previously published biochemical analyses revealed that the leaves, the seed and the pulp from baobab tree are rich in nutrients [11-14]. Literature reviews on baobab provided information on the species taxanomy and pharmacology, distribution, utilization, agronomy, and phytochemistry [5,15,16], brought out information on baobab botany, ecology, origin, propagation, main uses, genetic improvement and especially its importance for nutrition and poverty alleviation. Literature review revealed a great variation in reported values of nutrient contents of baobab part which may be due to the quality of the sample, the provenance of the sample, The age of the sample, the treatment before analysis, the soil structure and its chemical composition [14]. This study is aimed at the effect of ethanolic leaf extract of Adansonia digitata in alloxan-induced diabetic male Wistar rats.
Materials and Methods
Sample collection
Fresh leaves of Adansonia digitata were collected from the natural habitat of Gidan Adamu in Wukari L.G.A Taraba State, Nigeria in the month of June 2018. The specimen was identified in the Herbarium Unit of the Department of Biological Sciences, Federal University Wukari, Nigeria and was dried at room temperature.
Experimental animals
Male Wistar rats of average weight 180 g were purchased from animal house of College of Health Science, Benue State University Makurdi. They were acclimatized for two weeks prior to the commencement of the experiment, kept at room temperature, and feed using broiler starter. They were weighed prior to the experiment.
Ethanolic extraction
One hundred grams (100 g) of pulverized sample each of leaf and stem bark were weighed into a plastic container and filled with 400 ml ethanol respectively, and was allowed to stand for 24hrs, thereafter, filtered with Whatman No. 1 filter paper, the filtrate was concentrated using rotary evaporator under reduced pressure and concentrates transferred into air-tight container and preserved in the refrigerator at (4 0C) prior to administration.
Alloxanization
The animals were fasted over-night prior to induction and diabetes was induced in the male Wistar rats by intraperitoneal administration of alloxan (150 mg/kg body weight). After 72 hours the animals were tested and confirmed to be diabetic. The blood glucose concentrations of the animals were determined weekly using a glucometer (Accu-Check Active). Animals with fasting blood glucose of 240mg/dl and above were considered diabetic and were used for the study.
Experimental design
Twenty-five (25) male Wistar rat were divided into five (5) groups consisting of five (5) animals each. Out of the five groups, four were made diabetic as described below:
Group 1: Normal control
Group 2: Diabetic control
Group 3: Diabetic, treated with Adansonia digitata leaves (100 mg/kg)
Group 4: Diabetic treated with Adansonia digitata stem bark (100 mg/kg)
Group 5: Diabetic treated with Glibenclamide (5 mg/kg)
Treatment was given to the rats orally
Fasting blood glucose determination
A drop of blood was collected through tail puncture from the over-night fasted rats on an assay strip and read using Accu-Check glucometer. This was carried out on weekly basis for 21 days.
Liver function test
The serum biochemical examination was carried out using Vitros DT 60 Ã Chemistry Analyzer and the following parameters were measured: Alanine aminotransferase (ALT), Aspartate aminotransferase (AST), Alkaline phosphatase (ALP), Bilirubin (BIL) and Potassium (K).
Determination of haematological parameters
The total Red blood cell count (RBC), haemoglobin(HGB) concentration, White blood cell (WBC) count and Platelet count were determined using Abacus 280 auto hematology analyzer in General hospital Gboko, Benue state.
Statistical analysis
Statistical analysis was carried out using SPSS version 23 for the analysis of variance (ANOVA). Differences within the groups are considered statistically significant at (p<0.05) confidence level.
Results
Liver function test of diabetic rats treated with the leaves and stem bark of Adansonia digitata L. (Baobab) ethanolic extract
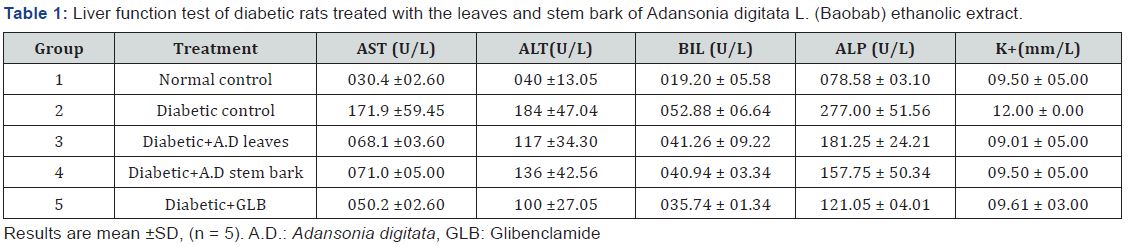
The result showed significant (p< 0.05) increase in the AST, ALT, ALP activities and bilirubin concentration in the diabetic control (group 2). Treatment with the leaf and stem bark extract caused significant decrease in in the levels of these parameters compared to the diabetic control as well as the normal control. There was no significant (p< 0.05) increase/decrease between the effect elicited by the standard drug and the extracts considering the same parameters. The result of K+ shows no significant increase in the level of K+ in the diabetic control (group 2) compared to the normal control (group 1). Treatment with the leaves and stem bark extract caused no significant decrease in the K+ level compared to the diabetic control. There was no significant increase/decrease between effects elicited by the standard drug and the extracts (Table 1).
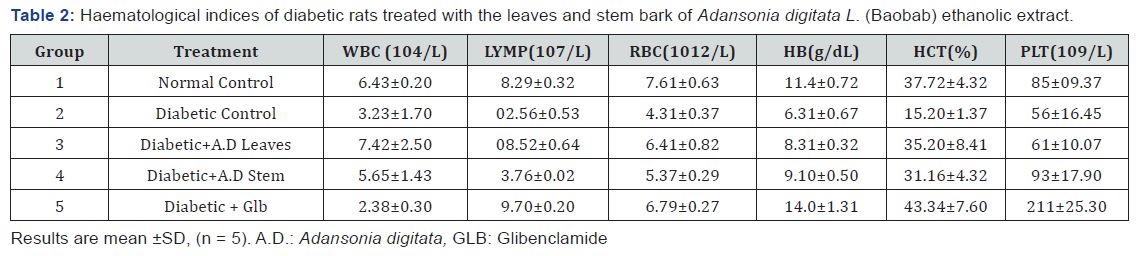
Hematological indices
The result of WBC and RBC showed a significant (p<0.05) decreases in the level of WBC and RBC in the diabetic control (group 2) compared to the normal control (group1). Treatment of diabetic rats with the extracts caused significant (p<0.05) increases in the levels of these parameters compared to the diabetic control. There was no significant decrease between effects elicited by the glibenclamide and the extracts. Similar pattern of observation was made for the lymphocyte concentration. The result of HCT shows a significant decrease in the level of HCT in the diabetic control (group 2) compared to the normal control (group 1). Treatment with the leaves and stem bark extract caused a significant (p>0.05) increase in the HCT level compared to the diabetic control. There was a significant (p>0.05) increase between effects elicited by the standard drug and the extracts.
The result of PLT, HB and HCT shows a significant decrease in the level of PLT, HB and HCT in the diabetic control (group 2) compared to the normal control (group1) and the test groups. Treatment of the diabetic rats with the extracts caused significant increase in the in the concentrations of these parameters compared to the diabetic control. However, the stem bark extract showed more potency considering these parameters (Table 2).
Changes in body weight of rats
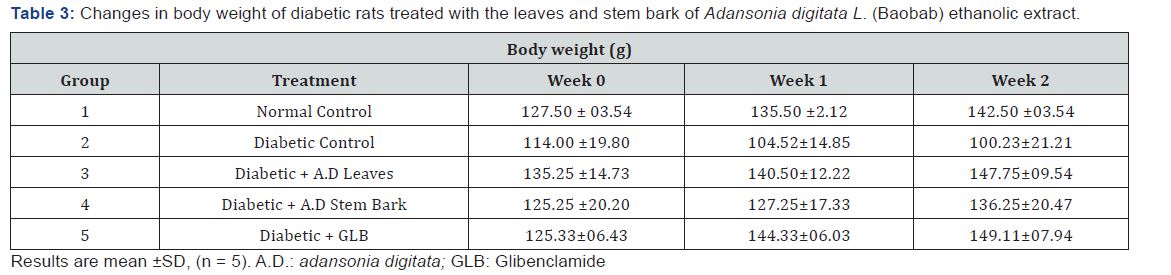
There was a significant (p>0.05) increase in weight of rats in all the groups during the period of the experiment except group 2 (diabetic control) which had decrease in body weight across the weeks (Table 3).
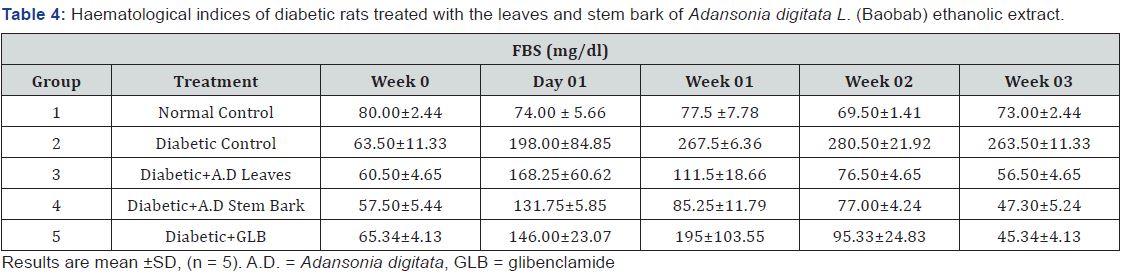
The result of FBS shows a significant increase in the diabetic control (group 2) compared to the normal control (group 1). Extract treatment shows significant (p<0.05) decrease in the FBS levels across the weeks compared to the diabetic control. There was no significant increase/decrease between the effect elicited by the standard drug and the extracts (Table 4).
Discussion
Medicinal plants are widely used by the populations of underdeveloped countries as alternative therapy. In Africa, hundreds of plants are used traditionally for the management and control of diabetes mellitus. Unfortunately, only a few of such African medicinal plants have received scientific scrutiny. Alloxan induces diabetes in experimental animals by destroying the beta cells of the Islet of Langerhans in the pancreas leading to reduction in the synthesis and release of insulin thereby inducing hyperglycemia [17]. Alloxan has been shown to induce free radical generation and cause tissue injury. The pancreas is especially susceptible to action of alloxan-induced free radical damage. Therefore, alloxan- induced diabetes is one of the frequently used models for the study of insulin dependent diabetes mellitus (IDDM) in experimental animals [18]. Insulin mediation of glucose intake by the cells is a critical step in maintaining glucose homeostasis and in clearing the postprandial glucose load [19,20]. Historical records provide a reservoir of basic information on the use of traditional medicine in the management of diabetes mellitus with plant extracts [21-25]. One of such part is the stem of Adansonnia digitata in the management of Alloxan- induced Diabetes mellitus in rat.
Preliminary phytochemical screening of the extract revealed the presence of flavonoids, tannins, saponins, cardiac glycosides, reducing sugars, glycosides, steroids and triterpenes [26]. The results of this study showed that the extract at all doses caused a significant (p<0.05) decrease in the blood glucose levels in Alloxan- induced diabetic Wistar rats. The mechanism by which the extract exerted the hypoglycemic effect appear to be related to the presence of flavonoids among other secondary metabolites or bioactive chemical constituents found in the plant extracts which may be an active constituent in a group or as an individual responsible for the hypoglycemic activity of the plant extract [26].
Flavonoids have been shown to exert their antioxidant activities by scavenging or quenching free radicals or by inhibiting enzymatic systems responsible for free radical generation [27]. Apart from being antioxidants, flavonoids have been reported to inhibit sodium-dependent vitamin C transporter 1 (SVCT 1) and glucose transporter Isoform 2 (Glut 2), the intestinal transporters for vitamin C and glucose, leading to a decrease in the intestinal absorption of glucose, hence decrease in the blood glucose concentration [26]. Several researchers have also demonstrated that flavonoids act as reducer of hyperglycemia by causing inhibition of renal glucose reabsorption through inhibition of the sodium- glucose symporters located in the proximal renal convulated tubule [27]. This also may probably be a possible mechanisms by which the plant extract exert its hypoglycemic effects in the diabetic animals and lend credence to the use of this plant in the management of diabetes mellitus.
Though hypoglycaemic potential of Adansonia digitata stem bark extract in alloxan-induced diabetic Wistar rats has been established, there is paucity of information about the anti hyperglycaemic effect of the leaves widely consumed and used in the management of diabetes mellitus in Hausa land, Nigeria. Intestinal sodium glucose transporter-1 (SGLT-1) was suggested to be involved in the absorption of quercetin glucosides [28]. Hence, they competitively inhibit sodium (Na+) dependent mucosal uptake of the non-metabolisable glucose analogue methyl- α-D-glucopyranoside via SGLT-1 using rat mid-jejunum, whereas quercetin (aglycone) and rutin had no effect [28]. Similarly, conjugated flavanoids such as Quercetin-3-Glycosides have the tendency of inhibiting Na+-independent, non-saturable uptake of glucose by SGLT-1 [28]. Flavonoids and glycosides also stimulate the secretion of insulin in β-cells of pancreas [29].
The detection of flavonoids in the extract can also be linked to the blood glucose lowering property by inhibiting intestinal absorption. Furthermore, flavonoids inhibit glucose-6-phophatase activity in the liver thereby suppressing gluconeogenesis and glycogenolysis and consequently reduce the hyperglycaemia. Many reports on herbal remedy for diabetes show that flavonoids exhibit anti-oxidant properties. The free radical scavenging ability of many flavonoids-containing extracts has been postulated as the mechanism which affords relief in many distressful diseased conditions of the body such as diabetes mellitus.
Hematological and biochemical indices have been reported to be a reliable parameter for assessment of health status of animals. Adansonia digitata extracts produced a significant fall in elevated levels of haematological parameters in diabetic rats. It has been reported that insulin deficiency occur in alloxan-induced diabetic rats, leading to alteration in the glucose metabolism such as elevated blood glucose and reduced level of insulin. In this study however, it was observed that Adansonia digitata extracts reversed the effect and tends to bring about normalcy in the parameters, this is compared with the observation with anti-diabetic drugs (glibenclamide) in diabetic rats and non-diabetic rats. It has also shown that there is a significant increase in weight of the various groups as shown in Table 3.
Conclusion
The result obtained in this study indicated that A. digitata leaves and stem bark extracts possess the potential to be used in the management of diabetes mellitus by being able to reverse the effects of alloxanization as elucidated by the different parameters under consideration. The presence of certain phytochemicals such as flavanoids, alkaloids, saponins, glycosidase and phenolics, may have overcome the negative effect of high carbohydrate content. Flavanoids in leaves have the potential of reducing intestinal absorption of glucose, inhibit carbohydrate digestion and increase hepatic activity of glucokinase thereby resulting into an increase in the pancreatic secretion of insulin from islet of langerhans.
References
- Okigbo RN, Mmeka E C (2008) Antimicrobial effects of three tropical plant extracts on Staphylococcus aureus, Escherichia coli and Candida albicans. Afri J Compl Alter Med 5(3): 226-229.
- Shoback DG, Gardner D (2011). Greenspan’s basic & clinical endocrinology. (9th edn). New York: McGraw-Hill Medical.
- De Caluwé E, Halamová K, Van Damme P (2010) Adansonia digitate L. A review of traditional uses, phytochemistry and pharmacology. Afrika focus 23: 11-51.
- Gebauer J, El-Siddig K, Ebert G (2002) Baobab (Adansonia digitata L.): a Review on a Multipurpose Tree with Promising Future in the Sudan. Gartenbauwissenschaft 67: 155-160.
- Addy EO, Eteshola E (1984). Nutritive value of a mixture of tigernut tubers (Cyperus esculentus L.) and baobab seeds (Adansonia digitata L.). Journal of the Science of Food and Agriculture 35(4): 437-440.
- Verrotti A, Scaparrotta A, Olivieri C, Chiarelli F (2012) Seizures and type 1 diabetes mellitus: current state of knowledge. Eur J Endocrinol 167(6): 749-758.
- Venter F, Venter J (1996) Baobab in Making the most of indigenous trees. Briza publications, Pretoria, South Africa, pp. 26-27.
- Sidibe M, Williams JT (2002) Baobab. Adansonia digitata. Fruits for the Future. International Centre for Underutilised Crops, Southampton, UK. p. 96.
- Yakubu OE, Imarenezor EPK, Udeh SMC (2016) Total antioxidant capacity, phenolic and flavonoids contents of partially purified aqueous extract of Vitex doniana leaves. FUW Trends in Science and Technology Journal 1(1): 211-214.
- Diop AG, Sakho M, Dornier M, Cisse M, Reynes M (2005) Le baobab africain (Adansonia digitata L.): principales caractéristques et utilisations. Fruits 61(1): 55-69.
- Chadare FJ, Linnemann AR, Hounhouigan JD, Nout MJR, Van Boekel MAJS (2009) Baobab Food Products: A Review on their Composition and Nutritional Value. Crit Rev Food Sci Nutr 49(3): 254-274.
- Szkudelski T (2001) The mechanism of Alloxan and Streptozocin action in β cell of the rats pancreas. Physiol Res 50(6): 536-546.
- Yakubu OE, Nwodo OFC, Joshua PE, Ugwu MN, Odu AD Okwo F (2014) Fractionation and determination of total antioxidant capacity, total phenolic and total flavonoids contents of aqueous, ethanol and n-hexane extracts of Vitex doniana leaves. African Journal of Biotechnology 13(5): 693-698.
- Shi Y, Hu FB (2014) The global implications of diabetes and cancer. Lancet 383(9933): 1947–1948.
- Bailey CJ, Day C (1989) Traditional plant medicines as treatments for diabetes. Diabetes Care 12(8): 553-564.
- Swanston-Flatt SK, Flatt PR, Dasy C, Bailey CJ (1991) Traditional dietary adjuncts for the treatment of diabetes mellitus. Proc Nutr Soc 50(3): 641-651.
- Gray AM Flatt PR (1999) Insulin-releasing and insulin-like activity of the traditional antidiabetic plant Coriander sativum (coriander). Br J Nutr 81(3): 203-208.
- Srinvasan K (2005) Plant foods in the management of diabetes mellitus: Spices as beneficial antidiabetic food adjuncts. Int J Food Sci Nutr 56(6): 399-414.
- Marles JR, Farnsworth NR (1995) Antidiabetic plants and their active constituents. Phytomedicine 2(2 ): 137-189.
- Lukacinova A, Mojzis J, Benacka R, Keller J, Maguth T, et al. (2008) Preventive effects of flavonoids on alloxan-induced diabetes mellitus in rats. Act Vet Brno 77(2): 175-182.
- Cermak R, Landgraf S, Wolffram S (2004) Quercetin glucosides inhibit glucose uptake into brush-border-membrane vesicles of porcine jejunum. Br J Nutr 91(6): 849-855.
- Hii CST, Howell SL (1985) Effect of flavonoids on insulin secretion and Ca2+ handling in rats islets of langerhans. J Endocrinol 107(1): 1-8.
- Grams J, Garvey WT (2015) Weight Loss and the Prevention and Treatment of Type 2 Diabetes Using Lifestyle Therapy, Pharmacotherapy, and Bariatric Surgery: Mechanisms of Action. Curr Obes Rep 4(2): 287–302.
- Tiwari AK (2004) Antioxidants: New generation therapeutic base for treatment of polygenic disorder. Current Science 86(8): 1092-1102.
- Ohaeri CC, Eluwa MC (2011) Abnormal biochemical and hematological indices in trypanomiasis as a threat to herd production. Vet Parasitol 177(): 199-202.
- Song J, Kwon O, Chen S, Daruwala R, Eck P, et al. (2002) Flavonoid inhibition of Sodium dependent Vitamin C transport 1 (SVCT 1) and Glucose Transporter Isoform 2 (GLUT 2), intestinal transporters for vitamin C and glucose. JBC 277(18): 15252-15260.
- Cheng J, Zhang W, Zhang X, Han F, Li X, et al. (2014) Effect of angiotensin- converting enzyme inhibitors and angiotensin II receptor blockers on all-cause mortality, cardiovascular deaths, and cardiovascular events in patients with diabetes mellitus. JAMA Intern Med 174(5): 773-785.
- Serena G, Camhi S, Sturgeon C, Yan S, Fasano A (2016) The Role of Gluten in Celiac Disease and Type 1 Diabetes. Nutrients 7(9): 7143–7162.






























