Metallic Biomaterials
Fehim Findik*
Subu-Technology Faculty, Sakarya Applied Science University, Turkey
Submission: September 17, 2018;Published: October 12, 2018
*Corresponding author: Fehim Findik, BIMAS-RC, Sakarya Applied Science University, Sakarya, 54187, Turkey.
How to cite this article: Fehim Findik. Metallic Biomaterials. Curr Trends Biomedical Eng & Biosci. 2018; 17(1): 555953. DOI:10.19080/CTBEB.2018.17.555953.
Keywords: Biomaterials; Metals; Titanium; Stainless steel; CoCr alloys
Introduction
Due to their superior electrical and thermal conductivity and mechanical properties, metals are used as biomaterials. Some metals are used as an alternative to hard tissue replacement, such as total hip and knee joints. Some metal alloys are used for active roles in devices such as vascular stents, catheter guide wires and cochlear implants. “Vanadium steel” used as a base metallic alloy for human use for bone fracture plates and screws. Most metals (eg Fe, Cr, Co, Ni, Ti, Nb and Mo) are used for alloys used to make implants, which can only be accepted by the body in small quantities.
Stainless Steels
The main stainless steel used for implant production was 18-8 (type 302), which is harder than vanadium steel and more resistant to corrosion. After 18-8sMo stainless steel, it was revealed that it contained a small percentage of molybdenum to increase corrosion resistance in salt water. This alloy was known as 316 type stainless steel. The minimum effective chromium concentration to report corrosion resistance in stainless steels is 11%. Chromium is a reactive element, but alloys can be passivated with 30% nitric acid to provide excellent corrosion resistance [1]. Especially austenitic 316 stainless steels are widely used for implant production. They cannot be hardened by heat treatment but can be hardened by cold working. These stainless steels are not magnetic and have better corrosion resistance than others. The presence of molybdenum increases the resistance to pitting corrosion in salt water.
CoCr Alloys
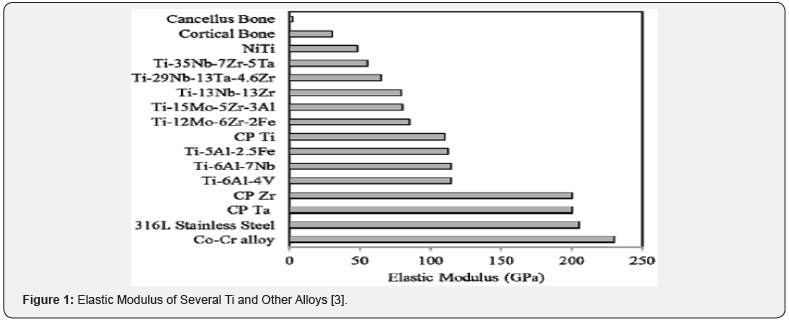
Two types of CoCr alloys (castable CoCrMo and forgeable CoNiCrMo) have been used as biomaterials. Especially castable CoCrMo alloy has been used in dentistry for the last 20 years and recently in artificial joints. The forged CoNiCrMo alloy is quite new and is now being used to fabricate the bodies of prostheses for severely loaded joints, such as knees and buttocks. Cast CoCrMo, forged CoCrWNi, and forged CoNiCrMo alloys are mentioned in ASTM documents for surgical implant use. At present only two are commonly used in implant manufacture, cast CoCrMo and forged CoNiCrMo alloys. Due to wear, corrosion and friction metallic products, the prosthesis can damage organs and local tissues. In vitro investigations have determined that particle Co is toxic to human osteoblast-like cell lines and prevents collagen, osteocalcin and alkaline phosphatase synthesis in culture medium. However, particulate Cr and CoCr alloys are well met by cell lines that do not have significant toxicity [2]. With changes in ultimate tensile strength the elastic modulus of CoCr alloys does not change. Values between 220 and 234 GPa, which are higher than other materials such as stainless steel. While this may have some indication of different load transfer modes to the articular joint equivalent bones, the improved modular implants are not immune and resistant to durability.
Ti Alloys
Titanium is suitable for implant use due to lighter weight (4.5 g / cm3) and good mechanochemical properties. There are three types of unalloyed commercially pure (cp) titanium for surgical implant use. Pollutants separate them; oxygen, iron and nitrogen should be measured carefully. Oxygen has an extreme effect on ductility and durability in particular [3]. To produce implants and chemical welds the Ti6Al4V alloy is generally used. The main alloying elements of the alloy are aluminum and vanadium. The Ti6Al4V alloy has approximately the same fatigue strength (550 MPa) of the CoCr alloy and then the rotary bending fatigue tests. Titanium is an allotropic material seen as a hexagonal close-up structure with a temperature of 882°C and a body-centered cubic structure over this temperature. Titanium alloys are strengthened and mechanically characterized by controlled composition and thermomechanical processing applications [4].
The elastic modulus of the Ti alloys is around 110 GPa except for the 13Nb13Zr alloy (Figure 1). The higher contamination content of Cp-Ti results in higher strength and lower ductility. The strength of the material differs from the 316 stainless steel or much lower than the CoCr alloys by a value much better than the tempered 316 stainless steel of the cast CoCrMo alloy.
Conclusion
Many metals, ceramics, polymers and composites can be used as biomaterials. In this mini-review, only stainless steel, CoCr and Ti alloys are summarized in metallic biomaterials [5].
References
- ASTM (1980) Annual Book of ASTM Standards. Philadelphia, USA.
- Schmalzried TP, Peters PC, Maurer BT, Bragdon CR, Harris WH (1996) Long-duration metal-on-metal total hip arthroplasties with low wear of the articulating surfaces. J Arthroplasty 11(3): 322-331.
- Yılmaz E, Gökçe A, Findik F, Gulsoy HÖ (2017) Characterization of biomedical Ti-16Nb-(0–4) Sn alloys produced by Powder Injection Molding. Vacuum 142: 164-174.
- Yılmaz E, Gökçe A, Findik F, Gulsoy HÖ (2017) Assessment of Ti– 16Nb–xZr alloys produced via PIM for implant applications. Journal of Thermal Analysis and Calorimetry 134(1): 7–14.
- Yılmaz E, Gökçe A, Findik F, Gulsoy HÖ (2018) Metallurgical properties and biomimetic HA deposition performance of Ti-Nb PIM alloys. Journal of Alloys and Compounds 746: 301–313.






























