Interaction of Saliva with Cobalt-Chromium-Based Dental Alloys in Casted Prosthetic Pieces
Willi Andrei Uriciuc1, Horatiu Vermesan2, Bianca Adina Bosca3* and Aranka Ilea1
1Department of Oral Rehabilitation, Oral Health and Dental Office Management, University of Medicine and Pharmacy Cluj-Napoca, Romania
2Corrosion and Anticorrosion Protection Laboratory, Technical University Cluj-Napoca, Romania,
3Department of Histology, Faculty of Medicine, University of Medicine and Pharmacy Cluj-Napoca, Romania
Submission: April 25, 2018; Published: May 08, 2018
*Corresponding author: Bianca Adina Bosca, Department of Histology, Faculty of Medicine, "Iuliu Hafieganu” University of Medicine and Pharmacy, Cluj-Napoca, Romania, Tel:+40740248923 ; Email:How to cite this article: Uriciuc W A, Verme.an H, Bo.ca A B, Ilea A. Interaction of Saliva with Cobalt-Chromium-Based Dental Alloys in Casted Prosthetic Pieces. Curr Trends Biomedical Eng & Biosci. 2018; 14(2): 555882.DOI:10.19080/CTBEB.2018.14c.555882
Abstract
Cobalt-chromium-based (CoCr) alloys are more frequently used in dental field compared with the precious metal alloys because they are cheaper, resistant to corrosion and compatible with dental ceramic that is used as aesthetic component of prosthetics. The physical properties of CoCr based alloys available on the dental materials market are variable, depending on the concentrations of elements such as tungsten (W) and molybdenum (Mo).
This study assessed the corrosion behavior and surface properties of five commercially available CoCr alloys with different contents of W and Mo. Firstly, the alloy casted samples were analyzed from the corrosion's point of view in artificial saliva (Ringer Solution). A current potential was applied to the surfaces for 120min, in an open circuit. Secondly, the specimens of dental casting alloys (CoCrMo, CoCrMoW and CoCrW alloys) were oxidized at high-temperature. All the samples were analyzed with a scanning electron microscope (SEM) and microscopic images of the oxide films on the alloys surface were captured.
The high content W alloys showed a linear and stable anticorrosion tendency in an open circuit current potential applied to the surface, in artificial saliva compared with the alloy without W, whose passive layer broke and recreated at the same potential. The SEM analyses showed that the CoCr-based alloys with 15% wt. content of W had created a stable oxide layer, with a higher density than the alloy without W.
In conclusion, the stability and resistance of oxide passive layer on CoCrW alloys surface had better anti-corrosion properties than the CoCrMo alloys. CoCr-based alloys with W content can be used as a single casted alloy for prosthetics dental structures, avoiding bimetallism and the corrosive effect in contact with saliva.
Keywords: Corrosion behavior; Surface properties; CoCr dental alloys; Tungsten content; Saliva.
Abbreviations: CoCr-based alloy: Cobalt-chromium-based alloy; Mo: Molybdenum; W: wolfram/tungsten; AS: Artificial Saliva; A: Aria; WE: Working Electrode; OCP: Open Circuit Potential; SEM: Scanning Electron Microscopy.
Introduction
Alloys have been used in medicine since 1900s in orthopedics, and also in dental medicine for their mechanical and physical properties. On the other hand for economic reasons, precious alloys (such as gold alloys) were seldom used, whereas nonprecious alloys use in dental field progressively increased. The main advantage of non-precious alloy is represented by the surface-passive oxide layers that provide the biocompatibility in the oral cavity and corrosion resistance [1]. The acid medium of the oral cavity and the changes in saliva composition influence the behavior of dental alloys surface in the oral cavity [2].
The problem concerning corrosion is represented by the products released in saliva when the materials are introduced in oral cavity as prosthodontics structures [3,4]. The commercial dental alloys that are used for casting the prosthetics parts have high biocompatibility properties [5].
CoCr-based alloys have been used in dental field for their corrosion resistance and for the compatibility with dental ceramic as aesthetic components [6]. At the beginning, CoCr- based alloy contained Mo (molybdenum) that provided a good fluidity of material for casting, but in time it was replaced or combined with W (wolfram/tungsten) that added a very good anti-corrosion behavior after casting the alloy in prosthetic pieces [7-9].
According to literature, the artificial saliva (AS) can be substituted with Ringer solution for testing the corrosion behavior of various alloys. It can be stored at room temperature and has pH of 6.8. This solution is also used for testing products in laboratories and also for medical therapy [10]. The aim of this work was to test the electrochemical behavior in artificial saliva and surface characterization after oxidation of five commercial CoCr-based alloys (with various contents of W and Mo).
Materials and Methods
The compositions of the five commercial CoCr-based alloys are presented in Table 1. The CoCr-based alloys purchased from the dental market were transformed in casted sample discs with aria (A) of 0,502 cm2 and cylindrical supports for electrical contact. Alloys that are named in Table 1 as 0-S (casted sample of alloy that contend 0% wt. W), 8-S (casted sample of alloy that contend 8% wt. W), 10-S (casted sample of alloy that contend 10% wt. W), 15-S (casted sample of alloy that contend 15% wt. W) and 16, 4-S (casted sample of alloy that contend 16.4% wt. W) were analyzed. The casted samples were immersed into Ringer Solution with the following composition: 8.6g NaCl, 0,33g CaCl2, 0,3g KCl, and water in balance.
The pieces were prepared for the electrochemical behavior tests as a working electrode (WE) in an electrochemical cell. The analysis was performed with Electrochemical Laboratory Voltalab PGZ100, and the results were processed with a Voltamaster 4 dedicated software. CoCr-based alloys working electrodes were prepared as casting discs. Moreover, we used a reference electrode (RE-Ag/AgCl), a counter-electrode (CE-Pt) and an electrolytic liquid (H2SO4 0,1N) at 23°C. The cell (Figure 1) was connected with VoltalabPGZ 100.
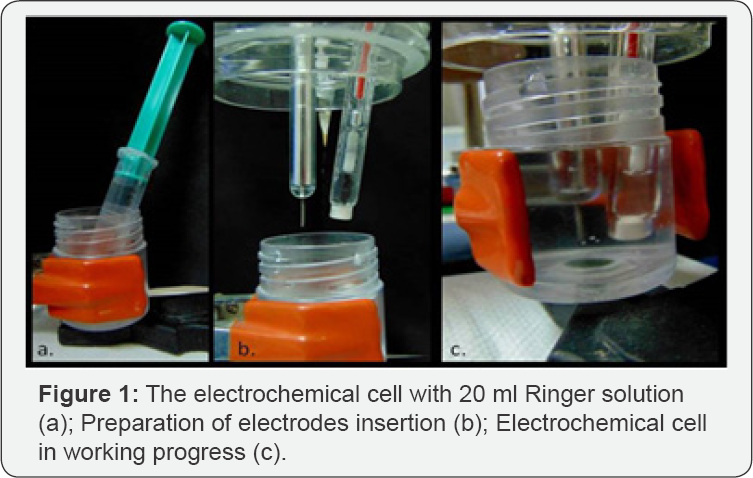
The CoCrMo alloy (0-S), CoCrMoW alloys (8-S, 10-S) and CoCrW alloys (15-S, 16,4-S) were tested in artificial saliva. The first test was performed for measuring the tendencies of the alloys to create a surface passive layer in electrolytic liquid, as anticorrosion response, in open circuit potential (OCP). The open circuit potential was measured for 120 minutes; the curve aspects and the tendency of potential to achieve 0 mV value were evaluated.
Secondly, the casted samples were sandblasted and subjected to mechanical finishing obtaining a mirror surface. Samples surfaces were heat oxidized in a P300 (Ivoclar Co.) programmable oven with vacuum set up capacities. Afterwards, the samples of casted alloys were prepared for Scanning Electron Microscopy (SEM).
Results
CoCrMo alloy open circuit potential curve was compared with the other two cronoamperometric curves for CoCrW alloys and CoCrMoW alloys (Figure 2).
The open circuit potential curves for all CoCr-based alloys ranged from negative potentials to tendencies of going to positive values. The evolution of electric potential in 120 minutes for each alloy showed the linearity and the anticorrosion trends. The values of electric potential (mV) applied in 120 minutes are presented in Table 2.
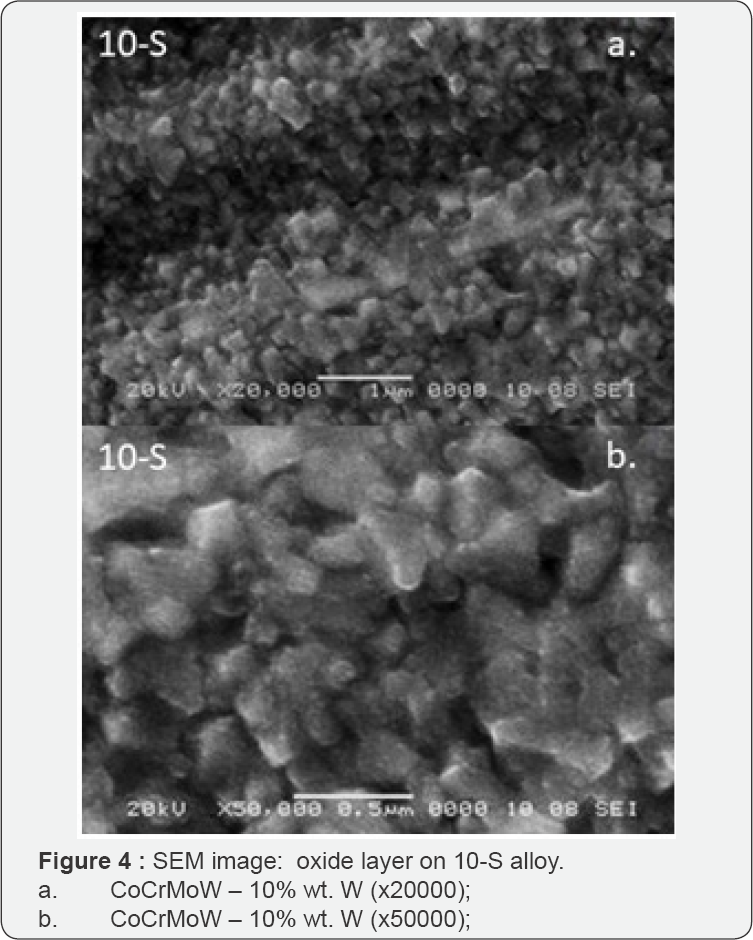
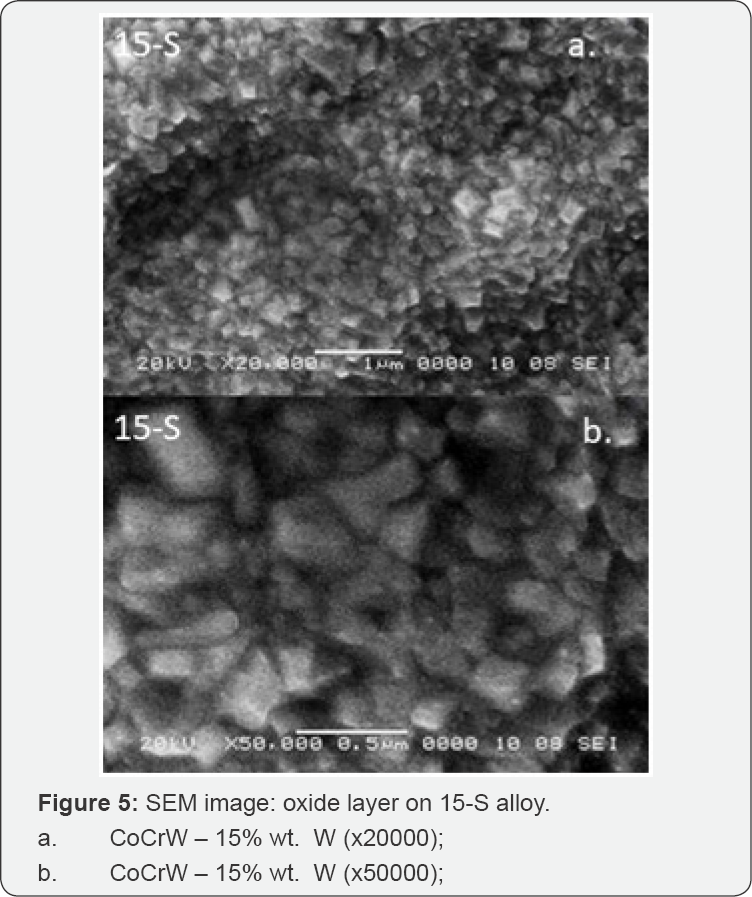
The different aspects of oxides passive layer formation can be seen in captured SEM images (Figure 3-5). SEM images revealed the density and topography of oxide passive layers that were created at the surface of three different CoCr based alloys after oxidation.

Discussion
Discussion begins with the following questions: Which CoCr- based alloy is optimum for casting dental precision ensembles? In a prosthetic ensemble, the stability of surface passive layer is very important in couple corrosion that could appear. Using one alloy for casting all the pieces in a prosthetic ensemble could avoid bimetallism.
Figure 2 showed that the chronoamperometric curve for CoCrW alloy with 15 wt. % of W (15-S) had higher tendency towards positive values, suggesting a better passive layer formation from the beginning of the electric potential application. The flat graph line revealed a constant current density value, indicating the absence of pitting corrosion and presence of generalized corrosion even at high current density. Moreover, the graph lines for CoCrMo alloy (8-S sample) and CoCrMoW alloy (10-S sample) had multiple peaks thus suggesting a pitting corrosion. These facts bring information regarding the alloys surface: the passive layers are dissolved and recreated over and over again when electric potential is applied.
The graph line for CoCrW alloy (16,4-S) was linear without peaks but more negative compared with the CoCrW alloy (15S); even the current potential difference in 120 minute was approximately the same (AE=165.2mV and AE=167.81mV respectively).
The SEM images provided important information for completing the electrochemical analyze. The gaps of the passive oxides layer on CoCrMo alloy were visible on the surface as black areas indicating the points where pitting corrosion could dissolve the alloy and release into the saliva the metallic components.
The highest density of the oxide layer was on the surface of the CoCr-based alloy with W content: 10- S and 15-S %wt. SEM images for 10-S and 15-S sample alloys showed no gaps, but an organized surface; these aspects were consistent with the electrochemical analysis which showed generalized corrosion and formation of a spinel stable oxide on the surface, without dissolution in saliva.
Therefore we can conclude that the optimum alloy for dental prosthetics must have electrochemical stability in saliva and the capacity to develop a passive oxide layer on the surface after the casting of structure pieces.
Conclusion
According to the value differences showed in the open circuit potential diagram, the CoCr-based alloys with W content were more stable in artificial saliva, compared with CoCr-based alloy with no W. From a kinetic point of view, CoCrMo alloys had tendencies to recreate the disrupted passive layer, whereas the CoCrW alloys were more resistant. The presence of the tungsten component in the alloy and its weight percentage make the difference by providing the quality of dental restorations and enhancing the material's resistance in human saliva.
Acknowledgment
This study was supported partially by the COFUND - ERA - HDHL ERANET Project, European and International Cooperation - Subprogram 3.2 - Horizon 2020, PNCDI III Program - Biomarkers for Nutrition and Health - "Innovative technological approaches for validation of salivary AGEs as novel biomarkers in evaluation of risk factors in diet-related diseases", no 25/1.09.2017.
References
- Geursten W (2002) Biocompatibility of dental casting alloys. Crit Rev Oral Biol Med 13(1): 71-84.
- Wataha JC, Lockwood PE, Khajotia SS, Turner RJ (1998) Effect of pH on element release from dental casting alloys. Prosthet Dent 80(6): 691698.
- Wataha JC (2000) Biocompatibility of dental casting alloys: a review. J Prosthet Dent 83(2): 223-234.
- vanNoort R (2013) Introduction to Dental Materials. (4th edn.), Elsevier Ltd.
- Yamanaka K, Mori M, Torita Y, Chiba A (2018) Effect of nitrogen on the microstructure and mechanical properties of Co-33Cr-9W alloys prepared by dental casting. J Mech Behav Biomed Mater 77: 693-700.
- Wang H, Feng Q, Li N, Xu S (2016) Evaluation of metal-ceramic bond characteristics of three dental Co-Cr alloys prepared with different fabrication techniques. J Prosthet Dent 116(6): 916-923.
- Yamanaka K, Mori M, Kuramoto K, Chiba A (2014) Development of new Co-Cr-E based biomedical alloys: Effects of micro alloying and thermo mechanical processing on microstructures and mechanical properties. Material&Design 55: 987-998.
- Barbieri M, Mencio F, Papi P, Rosella D, Di Carlo S, et al. (2017) Corrosion behavior of dental implants immersed into human saliva: preliminary results of an in vitro study. Eur Rev Med Pharmacol Sci 21(16): 3543-3548.
- Andrysenwicz E, Mystokowska J, Dobrowski R, Och E, Skolimowska K (2013) The influence of saliva and its substitutes on corrosion of some implant alloys. Acta Mechanica et Automatica 7(2): 69-74.
- Mareci D, Chelariu R, Sutiman D, Gordin DM, Gloriant T (2011) Evaluating electrochemical behavior of recrystallized titanium alloys in Ringer's solution. Mat Corr 62(12): 1117-1123.






























