The Cardio Kinesiograph System
Soumia Belouafa1*, Khalid Digua3, Brahim Sallek2 and Hassan Chaai3
1Laboratory of Biomolecules and Organic Synthesis, Faculty of Sciences Ben M’Sik Casablanca, Morocco
2Laboratory of Process and Environment Engineering, Faculty of Sciences and Technologies Mohammedia, Morocco
3Laboratory of Process Engineering, Faculty of Sciences Kenitra, Morocco
Submission: October 30, 2017; Published: January 19, 2018
*Corresponding author: Soumia Belouafa, Laboratory of Biomolecules and Organic Synthesis, Department of Chemistry, Faculty of Sciences Ben M’Sik Casablanca, Morocco, Email: sbelouafa@yahoo.fr
How to cite this article: Soumia B, Khalid D, Brahim S, Hassan C. Influence of Synthesis Parameters on the Chemical Composition and Physicochemical Characteristics of Oxygenated Calcium Phosphate Apatite. Curr Trends Biomedical Eng & Biosci. 2018; 11(1): 555802. DOI: 10.19080/ CTBEB.2018.11.555802.
Abstract
The main objective of the present work was to investigate the effect of synthesis parameters (temperature, maturation time, pH, Ca/P˩r ratio of reactants and composition of hydrogen peroxide) on chemical composition and physicochemical characteristics of oxygenated calcium phosphate apatite synthesized by precipitation from calcium nitrate Ca(NO3)2 and phosphoric acid H3PO4. The chemical composition of the products has been determined with chemical analysis and physicochemical characterizations of the samples have been carried out by X-ray diffraction (XRD) and infrared spectroscopy (IR).
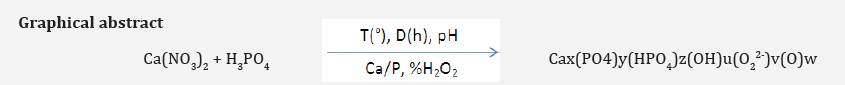
Keywords: Synthesis parameters; Oxygenated apatite; Chemical composition; Physicochemical characteristics
Introduction
The phosphocalcic oxygenated apatites are used as bone substitutes and as support matrices of antiseptic agents for many decades [1]. After implantation, they undergo a process of degradation and dissolution combined with bone neo-formation and progressive release of oxygenated species (O2, O22-); responsible for antiseptic properties [1]. We have developed, in a previous studies [2,3], an oxygenated apatite based of calcium phosphate by precipitation reaction from calcium nitrate (Ca(NO3)2) and orthophosphoric acid (H3PO4). However, the composition and crystal characteristics of apatites are, however, more difficult to control during the synthesis, mainly because of the ability of the lattice to accept substituent’s and vacancies [4]. The principal objective of this survey was to describe the effect of various parameters (temperature, maturation time, pH, Ca/P˩r ratio of reactants and composition of hydrogen peroxide) on chemical compositions and physicochemical characteristics of synthesized oxygenated apatites.
Experimental
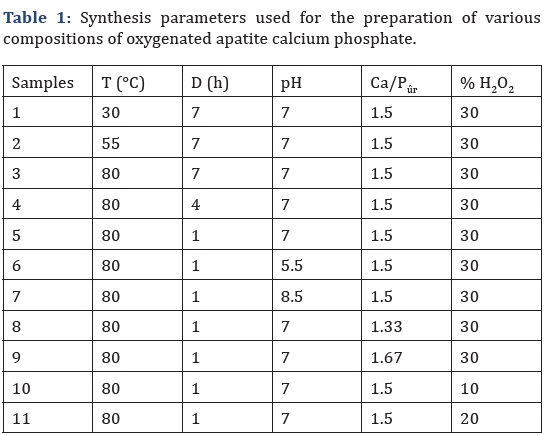
The synthesis method consists in mixing (500rpm) the calcium solution into 1L capacity reactor maintained at definite temperature. The pH was adjusted manually by addition of NH4OH solution (d=0.92). Then the phosphoric acid solution (3.875.10-2M) was poured into the reactor all at once and the reacting medium was kept under agitation at precise pH value. Finally, the suspension was vacuum filtered, washed with distilled water and dried in vacuo at room temperature. The specific parameters of each synthesis are summarized in Table 1.
The obtained products were analyzed by X-ray diffraction, infrared spectroscopy and by chemical analysis. X-ray diffraction analysis was carried out by means of a SEIFERT XRD 3000 P using CuK radiation. For infrared absorption analysis, 1mg of the powered samples was carefully mixed with 300mg of KBr and palletised under vacuum. The pallets were analysed using a Perkin Elmer 1600 FTIR spectrophotometer. Calcium, phosphorus and oxygenated species contents were determined by wet chemical methods: Calcium was titrated by complexometry [5]. The error on the calcium content is around 0.5%. Phosphorus content was analyzed by colorimetry [6]. The accuracy of this dosage was determined with a relative error of 0.5%. Molecular oxygen was determined by measuring the volume displaced during the acid dissolution of powder [1]. Uncertainty in this dosage is about 2%. Peroxide ions were titrated by manganimetry [7]. The relative error on this dosage is approximately 1%.
Results
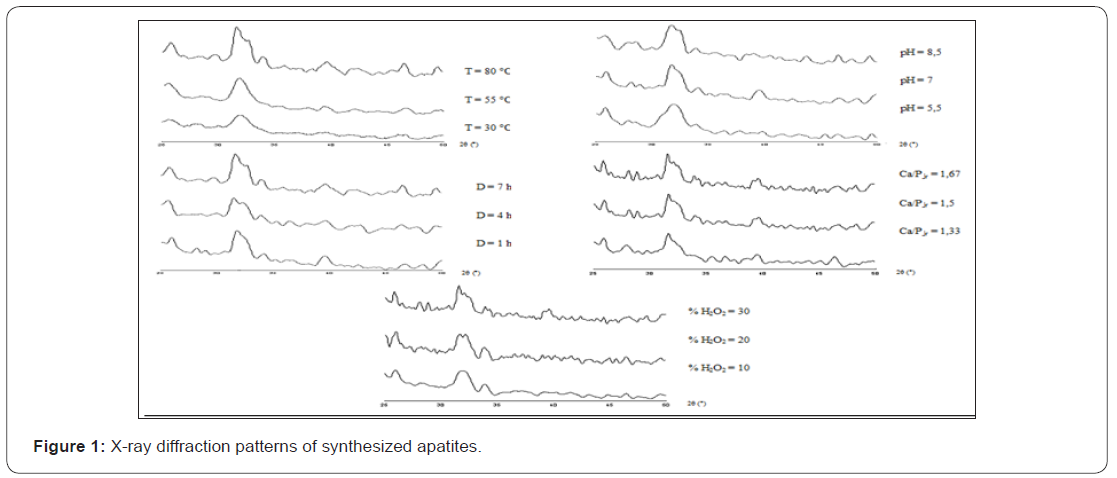
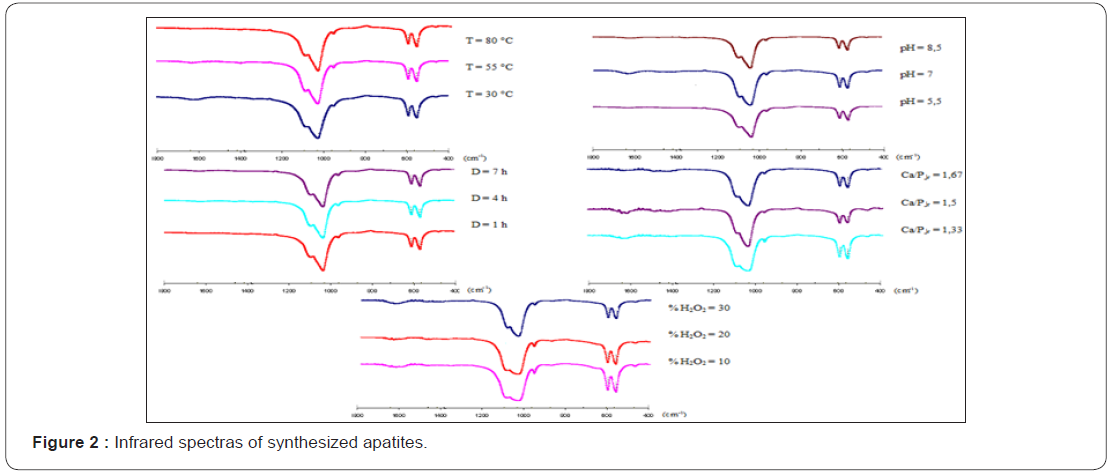
Figure 1 shows the X ray diffraction data of dried powders, while, Figure 2 illustrates the infrared spectra’s and Table 2 summarizes the chemical analysis. In result, we got the oxygenated apatites based calcium phosphate with different compositions and crystal characteristics and we examine successively the effect of each parameter:
Temperature
As shown by X-ray powder diffraction in Figure 1, the synthesis, conducted at 30 °C, provides an amorphous apatite while, at high temperatures, we got a more crystallized apatites. Chemical analysis (Table 2) showed, on the one hand, a positive correlation between temperature and rate of oxygenated species in obtained apatites, on the other hand, a negative correlation between the deviation from stoichiometry and the Ca/P ratio.
Maturation time
As can be seen in Table 2, the maturation time affect significantly the precipitate chemical composition. At first, the precipitate contains many peroxide groups, a few molecular oxygen and its Ca/P ratio is far from stoichiometry. Then, the peroxide groups quantity decreases and it was substituted by molecular oxygen. In the same way, our apatite approximates to the stoichiometry. This evolution is associated with refinement on X-ray diffraction patterns (Figure 1).
pH value
Chemical analysis of precipitated apatites at different pH value shows a negative correlation between pH value and deviation from stoichiometry (Table 2). So, at basic pH, Ca/P ratio of prepared apatite is near to the stoichiometry. Also, the infrared spectras of these compounds show a clear decrease of the HPO42- ions bands at basic pH (Figure 2). In contrast, oxygenated species composition occurs a significant positive correlation with pH value (Table 2).
Ca/Pr ratio
According to results presented in Table 2, the both ratios Ca/ Pr and Ca/P evolve in the same away, as well as the content of oxygen species.
H2O2 percentage
For different H2O2 concentrations, we noted here also a positive correlation between hydrogen peroxide concentration and oxygenated species content, as well as the Ca/P ratio (Table 2).

Discussion
According to our results, we can synthesize apatites with large specter, from poorly crystallized oxygenated apatites to greatest crystallized oxygenated one, and from deficient oxygenated apatites to like-stoichiometric oxygenated apatites. Similarly results was reported in previous studies [8-11], they indicate a dependency between the Ca/P ratio, % O2 and % O2 2- of synthesized apatites and the synthesis parameters (temperature, maturation time, pH, Ca/Pr and % H2O2). The Ca/P ratio raise was noted with temperature, maturation time, pH, Ca/Pr or % H2O2 increasing. In fact: The first formed precipitates are calcium deficient amorphous phosphates. Chow [12] have observed that in the first precipitation step, an amorphous calcium phosphate forms with Ca/P ratio ranged from 1.30 to 1.60. Boskeyet & Posner [13] have also observed that the formation of an apatitic phase is always preceded by the formation of an amorphous phase. Legeros [14] are prepared the hydroxyapatite, by precipitation reaction at 100 °C and pH ranging from 4 to 11, while at 60 °C and pH ranging from 5 to 6, they have detected the formation of octocalcic phosphate. This phenomenon was explained by Heughebaert [15] which showed that the amorphous phosphate conversion on apatitic phosphate is even faster than the temperature is higher. The pH of the reaction medium determines the concentration and the type of phosphate species acting during the precipitation. Indeed, the phosphoric acid is a tri-acid which the acidity constants for the various acid-base pairs, at 25 °C, are defined below [16]:
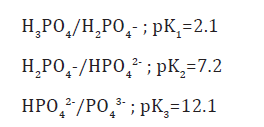
In the case of phosphoric acid (H3PO4) use in basic medium, the phosphorus is mainly present as PO4 3- and HPO42- ions. Thus, at basic pH, the formed apatites approximate to the stoichiometry. The higher Ca/Pr ratio means a reaction medium rich on Ca2+ and PO4 3- ions, which promotes calcium phosphates germination once precipitated and their evolution into a solid with higher Ca/P ratio in the expense of Ca2+ and PO4 3- free ions in solution. The evolution of Ca/P ratio, depending on H2O2 concentration, is in the same direction of evolution of oxygenated species content following the law of electronegativity of the apatite unit cell.
As for oxygenated species composition, all studied parameters seem influential: The decomposition of H2O2 was favored by increasing either the temperature or the pH of medium reaction which facilitates the oxygenated species incorporation in the apatitic structure and explains the elevation of oxygen species content in one of these cases. The elevation of the maturation time is, on the one hand, translates by the decomposition of peroxide groups present in the apatitic structure on molecular oxygen, and secondly, by the incorporation of new peroxide groups existing in solution [8]. This process occurs during the first hours of the evolution of precipitates which also correspond to the increase in the Ca/P ratio of solid phases as we have already mentioned. These two phenomena are probably related to a restructuring of deficient precipitated apatitic structure [8], which the mechanism is however not clarified. The increase of the oxygenated species content of solids, caused by the increase of Ca/Pr ratio, is directly related to the increase of Ca/P ratio of solids. Indeed, these two phenomena appear to be related to the charge-balance of the unit cell which must be neutral. The precipitation in the presence of hydrogen peroxide, of high concentration, promotes the increase of the oxygenated species content in the apatitic structure, which can be explained by the oversaturation of oxygenated species in the reaction medium.
Conclusion
The present report highlights the variations in chemical composition of oxygenated apatite based calcium phosphate according to studied synthesis parameters (Temperature, D, pH, Ca/P r and % H2O2). In fact, the Ca/P ratio and the oxygenated species content vary according to one or more of these parameters. Indeed, the Ca/P ratio of solids and oxygenated species insertion are highly favored by temperature, maturation time, pH, Ca/Pr ratio or the % H2O2. In sum, synthesis temperature, pH medium, Ca/Pr ratio and H2O2 concentration explain the effect of reaction medium on the oxygenated apatites composition while the maturation time seems to be more specifically related to the evolution of precipitates after their formation.
References
- Ledard C, Benque E, Lacout JL (1992) U.S. Patent n° 5 141 561.
- Belouafa S, Chaair H, Digua K, Sallek B, Essaadani A, et al. (2007) Synthesis, characterization and thermal behaviour of a Phosphocalcic Oxygenated Apatite. Journal of Advanced Materials 139-142.
- Belouafa S, Chaair F, Loukili H, K. Digua, Sallek B (2008) Characterization of antiseptic apatite powders prepared at biomimetics temperature and pH. J Materials Research 11(1): 93-96
- Gee A, Deitz VR (1953) Preparation, characterization and statistical studies of the physicochemical results of series of “b” carbonated calcium hydroxyapatites containing Mg2+ and CO2-3. Anal Chem 25: 1320-1324.
- Eanes ED, Meyer JL (1977) The maturation of crystalline calcium phosphates in aqueous suspensions at physiologic pH. Calcif Tiss Res 23(1): 259-269.
- Trombe JC, Montel GJ (1978) Inorg Nucl Chem 40: 15-21.
- Rey C (1984) Thèse d’Etat. Institut Nationale Polytechnique, Toulouse.
- Burgués J, Morales JG, Lopez-Macipe A (1999) Continuous Precipitation of Hydroxyapatite from Ca/Citrate/Phosphate Solutions using Microwave Heating. Crys Res Technol 34(5-6) : 757-762.
- Gomez-Morales J, Torrent-Burgués J, Boix T (2001) Precipitation of stoichiometric hydroxyapatite by a continuous method. Crys Res Technol 36(1): 15-26.
- Belouafa S, Faouzi F, Sallek B, Digua K, Zaoui F, et al. (2014) Etude de l’influence des paramètres de synthèse sur la composition chimique des apatites oxygénées carbonatées. J Phosphorus Sulfur and Silicone 189(9): 1-8.
- Chow LC, Eanes ED, Monogr (2001) Oral Sci 18: 130-147.
- Boskey AL, Posner AS (1976) Formation of hydroxyapatite at low supersaturation Physical Chemistry 80(1): 40-45.
- LeGeros RZ, Kijkowska R, LeGeros JP (1984) Formation and transformation of octacalcium phosphate, OCP: a preliminary report. Scan Electron Microsc 4(4): 1771-1777.
- Heughebaert JC (1977) Thèse de Doctorat. Institut National Polytechnique. Toulouse.
- Lucas S (2003) Thèse de Doctorat. Faculté des Sciences et Techniques. Limoges.
- Chaair H, Mansouri I, Nadir S (2001) Etude de la precipitation des phosphates de calcium. Phosphorus, Sulfur and Silicon 170(1): 247- 259.






























