On Generating Electro-Positive Drinking Water
Shimkevich AL*
NRC Kurchatov Institute, Russia
Submission: October 23, 2017; Published: November 08, 2017
*Corresponding author: Shimkevich AL, NRC Kurchatov Institute, Russia, Email: shimkevichal@nrcki.r
How to cite this article: Shimkevich A. On Generating Electro-Positive Drinking Water. Curr Trends Biomedical Eng & Biosci. 2017; 10(2): 555784 DOI: 10.19080/CTBEB.2017.10.555784.
Abstract
The electrochemical transformation of a drinking water into the positively charged one by means of the galvanic cell with the quasi-equilibrium anode and with the strongly polarized cathode is considered. These electrodes intensively produce hydroxyl radicals in the stream of pure water by the electric current in the cell. The mole fraction [OH] of the active and short-living radicals is nearly equal to the mole fraction [H3O+] of hydroxonium ions. These species form the positively charged pair as a hydrated cluster ()()+222nHOHOin liquid water. It is shown that such water is characterized by high concentration of the charged particles [(H2O)+2]~10-7 and by the shift of oxidation-reduction potential (ORP) into the positive region ORP > 1.0 V.
Mini Review
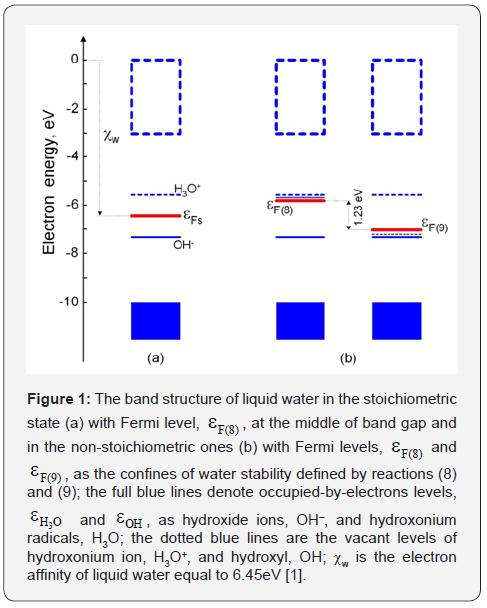
Simple water is described as a dielectric with the wide band gap, gε, equal to 6.9eV [1], It is the energy difference between the highest molecular orbital occupied by electrons and the lowest unoccupied molecular orbital. At the same time, there are two allowed local energy levels in the band gap of liquid water as occupied-by-electrons, εOH, hydroxide ion, OH–, and the vacant one, εH3O, of the hydroxonium ion, H3O+. These levels are located symmetrically nearby the band-gap middle with the energy difference between them of 1.75eV [2]. Such the model allows eliminating the inconsistencies of electrochemical properties of these well known charged particles [3] by means of the band theory of water [2].
So, one can use Fermi level, Fε, as an oxidation-reduction potential (ORP) [4,5] which indicates the tendency of liquid water to donate or accept the proton. When Fermi level is high, liquid water will donate protons, i.e. that is an antioxidant. Opposite, water will accept protons and will become the oxidant when Fermi level is low [6]. Since electrons cannot occupy the same quantum states, they have to occupy the ones that can accept them. If it is not happening, all the local quantum states (as oxidants) are inactive due to high Fermi level in the band gap of liquid water. For activating oxidizing agents, Fermi level is to shift down at least to the electron level of these agents and this is possible only when water becomes hyper-stoichiometric, H2O1–z (z < 0).
From this rule, one can easily get the difference between pH and ORP of liquid water. The first can be changed in stoichio metric water, H2O, by adding equivalent amount of anions and cations into liquid water. The second can be modified without changing pH by electro-oxidizing of pure liquid water. In as much as just a little non-stoichiometry of H2O1–z, is required (|z| < 10–10) for this and ORP will be sensitive to external conditions and may be changed without visible varying a composition of aqueous solution [6].
At the same time, such water can get the positive electrical charge that gives several unique properties of drinking water responsible for the health benefits: antibacterial and clearing action becomes curative. It is important to correctly estimate a scale of this tendency for generating electro-positive drinking water in practice. This estimate is the subject of the given work.
Non-Stoichiometry of Liquid Water
Allowed local electronic levels in the band gap of liquid water, εH3O and εOH, can be vacant and occupied by electrons like impurity levels in the band gap of solid dielectric. The molecular carriers of these levels are the attributive species of water as the occupied-by-electrons hydroxide ion, OH–, and the vacant one of hydroxonium ion, H3O+. They are obtained by the chemical reaction of dissociation [7]:
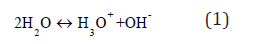
and their mole fractions [H3O+], [OH–] as the inherent water species satisfy the ratio [8]
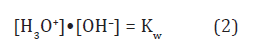
with the dissociation constant Kw =10–14 M2 at T =298 K. Their electronic levels, εH3O and εOH, are symmetrically to the band-gap middle which is Fermi level, Fsε, of stoi chiometric water, H2O, (Figure 1a) [2]. In non-stoi chiometric water, H2O1–z, Fermi level, εF , is the single-valued characteristic of ORP [5]:
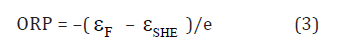
where e is the charge of electron and SHE ε = –6.21eV [2] is Fermi level of the Standard Hydrogen Electrode (SHE) [7]
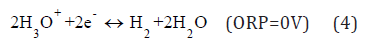
One can see that ORP becomes negative when Fermi level is shifted to the donor level, εH3O . Then, this level will be occupied by electrons forming the hydroxonium radicals, H3O, as strongest antioxidants. This transforms water into hypo-stoichiometric state, H2O1–z, (z>0). Opposite in the hyper-stoichiometric water (z<0), Fermi level is shifted to the acceptor level, εOH , making the positive ORP and forming hydroxyl radicals, OH, as strongest oxidants
So, Fermi level, εF(8) , in hypo-stoichiometric water H2O1–z (z>0) is defined by the electron population of the donor level, εH3O , as a molar fraction

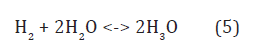
Fermi level, F(9) ε , in hyper-stoichiometric water (z<0) is defined by hole population of the acceptor level, εOH , as hydroxyl fraction,

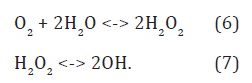
The states of liquid water in Figure 1b with Fermi levels: F(8) ε and F(9) ε are obtained by the standard reactions [7]:
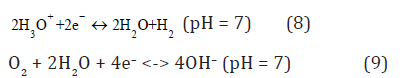
at [H3O+] = [OH–] = 10–7 M, [H2] = 1.6•10–3 M, [O2] = 2.7•10–4 M, H2 P = O2 P = 1 atm, T = 298 K and H2 K ~ 2•10–19 M, O2 K ~ 6•10–33 M, H2O2 K ~ 6•10–7 M [9] giving [H3O] •~ 2•10–11 M for the reaction (8) and [OH] ~ 8•10–13 M for (9) [2].
It means that the electron level, εH3O , is mostly vacant as hydroxonium ions, H3O+, due to F(8) ε is below εH3O (see Figure 1b, left) and the energy level, εOH , is fully occupied by electrons as hydroxide ions since this Fermi level is essentially above εOH . Fermi level, F(9) ε , is slightly above the electron level, εOH . Therefore it contains holes (the thin dotted blue line in Figure 1b, right) as hydroxyls (OH) but the energy level, εH3O , is fully vacant as hydroxonium ions, H3O+, since F(9) ε is essentially below εH3O .
Forcedly shifted Fermi level, εF , in the band gap of liquid water is determined rigorously by the ratios of mole fractions: [H3O+]/[H3O] and [OH]/[OH–], of the vacant species (H3O+, OH) and the occupied-by-electrons ones (H3O, OH–) for the energy levels, εH3O and εOH , in the band gap of liquid water. These ratios are given by Fermi–Dirac statistics which can be simplified to Maxwell–Boltzmann distribution of electrons and holes in the corresponding energy levels [10]:
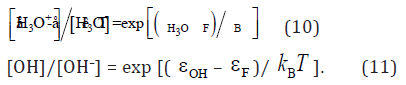
Then, submitting [H3O+], [H3O] of the electrode (8) into (10) and [OH], [OH–] of the electrode (9) into (11), we have obtained in [2]: εH3O – εOH = 1.75eV out of the well-known requirement of the chemical stability of liquid water: F(8) ε – F(9) ε = 1.23eV [7]. So, εH3O = –5.58eV and εOH = –7.32eV.
Thus, consideration of liquid water in the frame of band theory shows that varying its ORP is the immediate result of Fermi level shift in the band gap. Water becomes an antioxidant, i.e. hypo-stoi chiometric, H2O1–z, with z>0 when Fermi level is shifted to the level εH3O which is occupied by electrons and hydroxonium radicals (H3O) are formed. Opposite, in hyperstoichiometric water (z<0), Fermi level is shifted to the level εOH which is occupied by holes and forms hydroxyls. Therefore water becomes oxidant.
Now, we can obtain the dependence of Fermi level on the index, z

of non-stoi chiometric water, H2O1–z as the graph represented in Figure 2 [6].

One can see that the region of chemical water stability (-1×10-13≤z≤4×10-13 ) is shifted to the hypo-stoichiometric state, H2O1–z (z>0), which is achieved easier by least shifting of Fermi level from the band-gap middle to the donor level, εH3O , as well as the hyper-stoichiometric one, H2O1–z (z<0), is achieved by a little variation of z to the acceptor level, εOH . At the same time, Fermi level is shifted in the band gap about 1eV.
Electro-Oxidizing of Liquid Water
Forced oxidizing of liquid water (shifting εF down) can be carried out by the electrochemical cell with the voltage of ~ 2V between the strongly polarized cathode and the anode at quasiequilibrium with water [6] under obvious condition [11]:
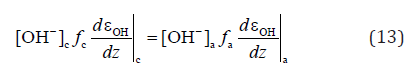
at fa >> fc . Here index “c” denotes cathode and “a” – anode, fi is the specific surface of distributed i-electrode, and

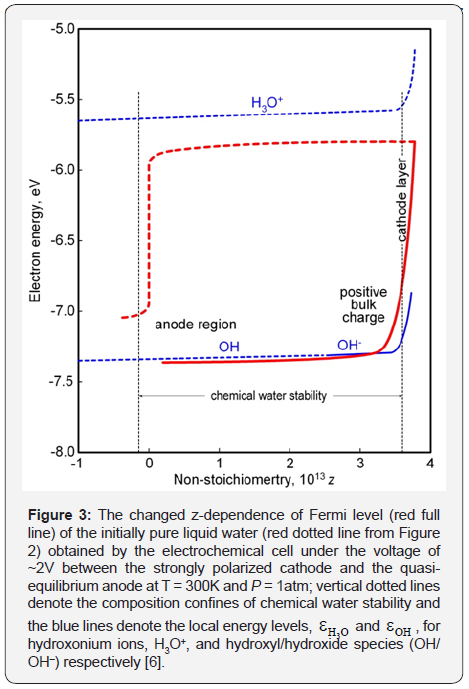
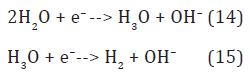
Hydroxide ions are discharged to hydroxyls by quasiequilibrium anodic reaction [12]
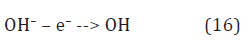
in the bulk anode region (Figure 3). Here, the hydroxonium radicals, H3O, diffused out of the cathode layer donate electrons to hydroxyl radicals by reaction
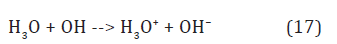
All this forms the positive charge in liquid water near the cathode (Figure 3) with the ratio
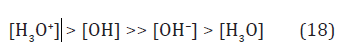
In contrast to (12) here, the index z of non-stoichiometric water, H2O1–z, is defined by formula [11]
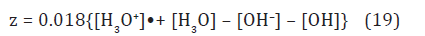
We have obtained in [11] the z-depended graphs of these species as shown in Figure 4. One can see the equal mole fractions of hydroxonium ions and hydroxyls.
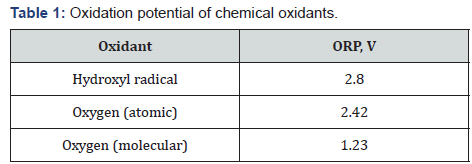
Thus, the ratio (18) indicates the strong acidic reaction of the chemically stable hypo-stoi chiometric water containing the large mole fraction of hydroxyls ([OH] ~ 10–7M) as strongest oxidants (see Table 1).
Oxidation potential of chemical oxidants [13] .
In this case, the hydroxyl radicals quickly form the solvated acceptors for electrons by reaction
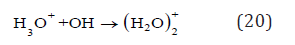
in the bulk of electrochemical cell due to their very short lifetime (only few nanoseconds) [8].

Conclusion
The quick response of Fermi level to changing of the water non-stoichiometry, z, takes place in the stoi chiometric state (z=0). Just therefore, a little additive of oxidant in pure water changes oxidation-reduction potential (Fermi level) appreciably. Such the effect may be gotten also by electric oxidizing of pure water in the electrochemical cell with strongly polarized cathode and the anode in equilibrium with the aqueous medium under the condition (13).
The advantages of this approach are the high efficiency of oxidation reaction, the simplicity of the procedure, low cost, and there is no need for special sorbents because pure water itself becomes the agent for oxidizing pollutants. Such water can contain the large concentration of the charged particles [(H2O)+2]~10-7 M and the oxidation-reduction potential can be in the positive region ORP > 1.0 V.
Acknowledgement
Author thanks the Russian Foundation of Basic Research (RFBR) for supporting this work (grant # 16-08-00029a) and appreciates his colleagues at active discussing all the aspects of electro-oxidizing water.
References
- Couto PCD, Guedes RC, Costa Cabral BJ (2004) The density of states and band gap of liquid water by sequential Monte-Carlo and quantum mechanics calculations 34(1): 42-47.
- Shimkevich AL (2014) Electrochemical view of the band gap of liquid water for any solution 4(4): 243-249.
- Do Couto PC (2007) Understanding electronic properties of water: a theoretical approach to the calculation of the adiabatic band gap of liquid water / Thesis for PhD degree. Lisbon University 4(4).
- Alekseev PN, Semchenkov YM, Shimkevich AL (2012) Aqueous nanofluid as a two-phase coolant for PWR. Science and Technology of Nuclear Installations.
- Shimkevich AL, Shimkevich IY (2012) On 2D water chemistry.
- Shimkevich AL (2017) On electrochemical managing the properties of aqueous coolant. Mod Chem Appl 5(1): 1000217(1-5).
- Bard AJ, Parsons R, Jordan J (1985) Standard Potentials in Aqueous Solutions (1st edn), Dekker M, New York, USA.
- Bandura AV, Lvov SL (2006) The ionization constant of water over wide ranges of temperature and density. J Phys Chem Ref Data 35(1): 15-30.
- Tables of Physical Constants.
- Kittel C (2004) Introduction to Solid State Physics.
- Shimkevich AL (2017) The concept for electrochemical cleaning of waste water.
- Shimkevich AL (2014) On performance capabilities of alkaline anolyte in wastewater management.
- Hannmann L, Powers K, Shepherd O, Taylor H (2012) Removal of ciprofloxacin from water with chemical oxidation.






























