Stem Cell Scaffolds for Tissue Engineering
Munni Shaik and Subramanyam G*
Director, Narayana Medical Institutions, India
Submission: September 05, 2017; Published: September 12, 2017
*Corresponding author: Subramanyam G, Director, Narayana Medical Institutions, Nellore, India, Email: director.nmch@gmail.com
How to cite this article: Munni S, Subramanyam G. Stem Cell Scaffolds for Tissue Engineering. Curr Trends Biomedical Eng & Biosci. 2017; 9(1): 555752. DOI: 10.19080/CTBEB.2017.09.555752
Review
Tissue engineering is the science that combines the principles of cellular biology, material science and biomedical engineering techniques in order to obtain biological substitutes for regenerating, replacing, modifying, repairing or restoring the function of organs and tissues. For stem cells to be used in tissue engineering a scaffold is essential to provide the necessary support for the transport of nutrients, oxygen and the elimination of metabolic waste, promoting a conducive environment for cell growth and differentiation. The development of new biomaterials for tissue engineering provides a scientific basis for the creation of scaffolds that could provide appropriate regeneration and tissue repair. Scaffolds can be classified as permanent or resorbable. Permanent scaffolds are stable in vivo, while the resorbable scaffolds are reabsorbed in vivo, metabolized by the body.
Biomaterials with potential application for tissue engineering
According to O’Brien (2011), these considerations are biocompatibility, biodegradability, mechanical properties consistent with the anatomical site that will receive the scaffold, an architecture that enables cellular penetration and nutrients diffusion, and the technology used should enable a small scale production [1]. Typically, three groups of biomaterials are used to manufacture scaffolds: ceramic, synthetic polymers, and natural polymers.
Natural polymers may be made manufactured from components found in the extracellular matrix, such as collagen, fibrinogen, hyaluronic acid, etc., having an advantage of being biocompatible, and having mechanical properties similar to natural tissue. They have low toxicity, low chronic inflammatory response, can be combined with other natural or synthetic materials. Its disadvantages include low mechanical strength, difficult to handle and need for chemical modification. E.g. Collagen, Fibrin, Platelet-rich plasma, chitosan, alginates, gelatin, elastin, Silk. The platelet concentrates are widely used in tissue regeneration because of its high content of growth factors and their easy processing. Acellular matrices are collagen rich biomaterials generated by isolating extracellular matrices from native tissues through the process of decellularization. These include small intestinal submucosa (SIS) and bladder acellular matrices (BAM), acellular amniotic membrane, acellular pericardium, and acellular dermal tissue.
Synthetic polymers can be manufactured with different times of degradation, mechanical properties, shape and porosity, but they have low cell adhesion, low ductility and low bioactivity. E.g. Polyglycolic acid, polylactic acid, and polylactic-co-glycolic acid [PGA, PLA, PCL].
Ceramics have biocompatible, similar to the inorganic component of the bone, osteo-conductive, absence of protein (absence of immune response) and long degradation time in vivo. However, they have poor mechanical properties. They are widely used in orthopedics and dentistry to repair bone defects, maintenance of the alveolar ridge and as well as orthopedic and dental implants. Disadvantage is inability to use on large bone defects which require stabilization. In such cases synthetic and natural polymers may be used to overcome these limitations. E.g. Hydroxyapatite,Tricalcium phosphate.
Stem cell culture and scaffold
Each tissue needs specific requirements, that will depend on the type of cell, the site, its function, and its mechanical properties. The application of stem cells in tissue engineering requires a large number of cells of high quality, which requires quick cell expansion. Two-dimensional (2D) cultures require stem cell growth to occur in monolayers atop stromal layers that supports stem cell proliferation or atop membranes with or without growth factors. Furthermore, 2D culture systems face difficulties in meeting the requirements of many downstream applications due to the inherent heterogeneity, limited scalability or reproducibility and incompatibility with the development of in vitro models that accurately simulate the native stem cell niche. Three dimensional (3D) culture can improve stem cell viability and function offering a higher degree of efficiency and consistency which makes more promising tool for preclinical research. The types of 3D stem cell culture systems include plate or culture dish, spinner flask, rotating wall vessel (RWV) and perfusion bioreactor system. Microcarrier systems can produce cells of better quality and purity due to it possesses good mass and gas diffusion properties. Alginate microencapsulates and thermoreversible hydrogel systems can protect the cells from shear force induced cell death. Nanostructure scaffolds composed of self-assembling peptides have the ability to form a biologically active matrix that displays functional motifs such as RGD (arginine-glycine-aspartic acid), BMHP1 (bone marrow homing peptide 1), and BMHP2 (bone marrow homing peptide 2).
Stem cell differentiation into organs and mekhanism
Although significant advances have been made recently in the development of artificial kidneys, pancreata, livers, cardiac muscle, skeletal muscle, and blood vessels [2], a better understanding of the cellular mechanisms that guide stem cell behavior in native and engineered 3D microenvironments would facilitate even greater progress. Stem cells respond to biophysical cues using cell signaling crosstalk, receptors and ligand interaction, protein modifications, protein-protein interactions, and transcriptional and translational regulations. Various mechanotransduction pathways have been proposed, such as the MAPK, the PI3K/Akt, RhoA/ROCK, Wnt/p-catenin, and the TGF-p pathways that rely mostly on the interaction of the cell with its biophysical environment. All these pathways coupled with many other potent growth factor-mediated signaling pathways to regulate stem cell fate.
Neural stem cells spontaneously differentiate into neurons when cultured on hydrogen terminated ultra-nanocrystalline diamond films with fibronectin integrin beat-1, focal adhesion kinase, and the MAPK pathway plays a decisive role [3]. Osteoblast differentiation on micro/nanotextured topography and titanium implant surface as well as magnesium alloy coated with porous b-tricalcium phosphate is modulated by the activation of the MAPK pathway [4,5]. Composite scaffolds of porous p-calcium silicate with poly-D,L-lactide-glycolide enhance the osteogenic and angiogenic potential of MSCs and endothelial cells by recruitment of AMP-activated protein kinase, Erk1/2 and PI3K/ Akt pathways [6]. High RhoA activity associated with high actomyosin contractility induces osteogenesis, while low RhoA activity leads to adipogenesis. Parekh et al. [7] have demonstrated that the osteogenic differentiation of bone marrow stem cells in 2D polyethylene glycol hydrogel in the absence of supplements is triggered through elevated expression levels of actin and myosin filaments. A dosage-dependent Wnt signaling results in either maintenance of the pluripotency or promotion of neural differentiation (Figure 1).

Stem cells in bladder tissue regeneration
Bladder is composed of four layers: the urothelium, lamina propria, muscularis propria, and serosa. Patients with neurogenic bladder often require bladder augmentation for small capacity or high intravesical pressures that threaten the upper urinary tract. For successful stem cell use in functional bladder bioengineering, it is crucial to achieve natural crosstalk between the transplanted stem cells and the scaffold, the host immune system and the existing bladder microenvironment, a full comprehension of which is still missing.
BAM and SIS matrices have been shown to be compatible with various urothelial and smooth muscle cell types and are used to study bladder regeneration in animal models. Nanofibrous poly (s-caprolactone)/poly(L-lactic acid) (PCL/PLLA) scaffolds have been shown to be biocompatible with adhesion of human urothelial and smooth muscle fiber cells. Electrospun PLLA nanofiber scaffolds have also been shown to support bladder smooth muscle growth and alignment.
In the last two decades many different natural (amniotic membrane, bladder acellular matrix, collagen, silk and synthetic poly lactic-co-glycolic acid (PLGA), polyurethane, poly- carbonate-urethane-urea) matrices have already found their application in preclinical and clinical bladder tissue engineering. In recent years the use of (multi-)layered hybrid scaffolds has been established, as such combinatorial approaches often result in a more specific, customized microenvironment for each cellular layer, an improved regeneration, and better biomechanical properties of the construct.
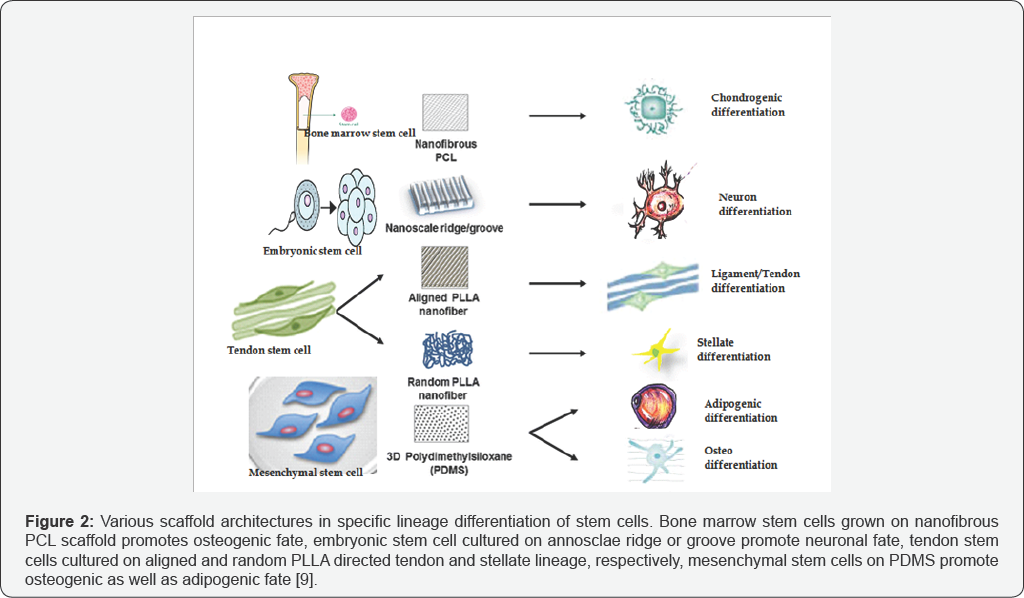
The first clinical study utilizing patients' own autologous cells in combination with collagen alone or a collagen-PGA hybrid constructs was published in 2006 (Figure 2) [8,9]. The bladder substitutes were seeded in vitro with urothelial and smooth muscle cells and implanted in young patients with myelomeningocele and end-stage bladder disease. After a mean follow-up of 46 months, the collagen-PGA composite resulted in a significantly improved bladder regeneration compared to collagen scaffold alone. An additional omental wrap enhanced the graft vascularization, further improving the transplanted cell survival and scaffold engraftment. Scaffold biopsy analysis showed proper architecture of the reconstructed bladder wall. However, only two patients showed increased bladder compliance and capacities and longer dry periods over time. The majority of treated patients lacked an improvement in bladder compliance and capacity and showed the development of fibrous tissue in their transplanted bladder walls. Although this study demonstrated the feasibility and safety of the transplantation technique, it requires further research to achieve a functional bladder equivalent.
Conclusion
Tissue engineering is a promising field that has been developing intensely due to its potential for clinical application in cellular maintenance/differentiation. Scaffold functionalization tuned for specific application and cell response is the targeted approach.
References
- O'brien FJ (2011) Biomaterials & scaffolds for tissue engineering. Materials today 14(3): 88-95.
- Gong Z, Niklason LE (2008) Small-diameter human vessel wall engineered from bone marrow-derived mesenchymal stem cells (hMSCs). The FASEB Journal 22(6): 1635-1648.
- Chen YC, Lee DC, Tsai TY, Hsiao CY, Liu JW, et al. (2010) Induction and regulation of differentiation in neural stem cells on ultrananocrystalline diamond films. Biomaterials 31(21): 5575-5587.
- Wang W, Liu Q, Zhang Y, Zhao L (2014) Involvement of ILK/ERK1/2 and ILK/p38 pathways in mediating the enhanced osteoblast differentiation by micro/nanotopography. Acta biomaterialia 10(8): 3705-3715.
- Jiang T, Guo L, Ni S, Zhao Y (2015) Upregulation of cell proliferation via Shc and ERK1/2 MAPK signaling in SaOS-2 osteoblasts grown on magnesium alloy surface coating with tricalcium phosphate. Journal of Materials Science: Materials in Medicine 26(4): 158.
- Wang C, Lin K, Chang J, Sun J (2013) Osteogenesis and angiogenesis induced by porous p-CaSiO 3/PDLGA composite scaffold via activation of AMPK/ERK1/2 and PI3K/Akt pathways. Biomaterials 34(1): 64-77.
- Parekh SH, Chatterjee K, Lin-Gibson S, Moore NM, Cicerone MT, et al. (2011) Modulus-driven differentiation of marrow stromal cells in 3D scaffolds that is independent of myosin-based cytoskeletal tension. Biomaterials 32(9): 2256-2264.
- Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB (2006) Tissue-engineered autologous bladders for patients needing cystoplasty. The lancet 367(9518): 1241-1246.
- Krishna L, Dhamodaran K, Jayadev C, Chatterjee K, Shetty R, et al. (2016) Nano structured scaffold as a determinant of stem cell fate. Stem cell research & therapy 7(1): 188.






























