Applications of LAMP Based Diagnostic Kits in Crop Management
Spandan Chaudhary and Prashanth G Bagali*
Diagnostics & Genomics Research, Xcelris labs Ltd., India
Submission: July 20, 2017; Published: August 21, 2017
*Corresponding author: Prashanth G Bagali, Diagnostics & Genomics Research, Xcelris labs Ltd., India, Email: prashanth.bagali@xcelrislabs.com
How to cite this article: Spandan C, Prashanth G B. Applications of LAMP Based Diagnostic Kits in Crop Management. Curr Trends Biomedical Eng & Biosci. 2017; 7(2): 555708. DOI: 10.19080/CTBEB.2017.07.555708.
Abstract
Pathogens such as bacteria, fungus and viruses are interminable factors responsible for food loss and plant infection. Advanced disease detection methods play a pivotal role in the prevention of damage to crop yield and ensuring minimum deprivation of plants during growth, harvest and storage. In this study, we have discussed the characteristic features of traditional methods of detection and identification of pathogens, namely, polymerase chain reaction (PCR), fluorescence in-situ hybridization (FISH), enzyme-linked immunosorbent assay (ELISA), immunofluorescence (IF), flow cytometry, colony forming unit, DNA-RNA based methods, enzyme and antibody based detection methods etc. All these methods require high-tech infrastructure and laboratory facility. In the present article, we have attempted to review the various applications of loop-mediated isothermal amplification (LAMP) technique and it's commercial benefits in the field testing of pathogens and plant diseases.
Keywords: LAMP method; Plant pathogens; Isothermal amplification technique; Traditional methods of detection of pathogens; Field testing
Introduction
Human population is increasing at exponential growth in all parts of the world. Based on the recent report on food security released in 2010 [1]. Requirement of food will increase due to continuous increase in the human population leading to 70% more food requirement by 2050 [1]. Vegetarian population of the world is estimated to be 1.5 billion in 2010 [2]. Apart from the humans all the feedstock animals also rely on food produced by the plants. The demand of food is higher than the supply because of continuous decrease in the agricultural land. Under such circumstance, effective crop management is essential. The crop losses due to pathogenic infection was around 20-40% [3]. Rice, wheat, barley, maize and soybean are majorly consumed crop globally which was reduced by 12% due to plant diseases. Whereas the crop yield of groundnuts and potatoes were affected around 24% and of wheat and cotton around 50% and 80% [4]. Economic losses due to pathogens in US only was estimated to be 40 billion dollars so worldwide loses can be imagined [5,6]. With the evolution of science, researchers have found numerous techniques to identify pathogens but all of them require high initial cost, hi-tech infrastructure facility and experienced researchers. Therefore, these technologies are confined to the laboratories or research institutes located in cities. There is an urgent need to develop and popularize technologies that are suitable to the rural areas for speedy testing of pathogens to avoid epidemics and for launching crop protection measures to manage pathogens [7-29].
Discussion
Laboratory based technologies for detection and identification of plant pathogens
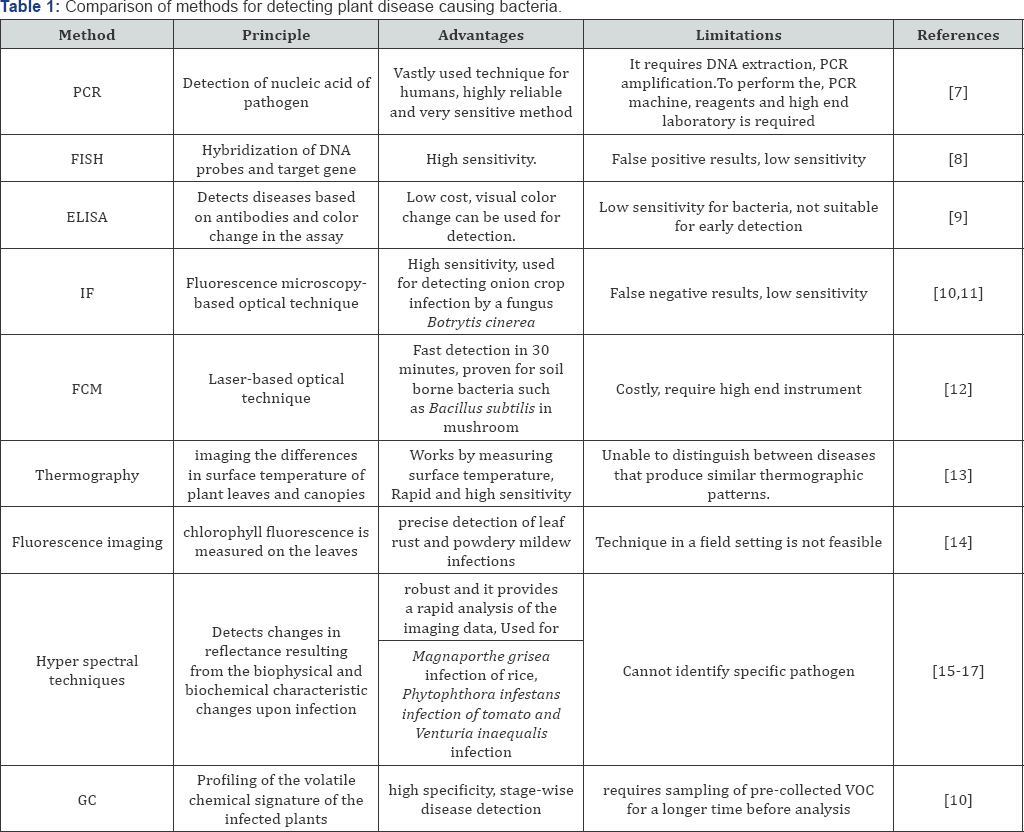
Identification of the pathogens plays a pivotal role in controling of plant diseases and in taking relevant crop protection measures Fang et al. [11], have divided the methods for pathogen detection in two categories: direct methods and indirect methods. Direct methods includes detection of pathogens through the molecules produced by them or by using their DNA/RNA etc. A comparison of direct pathogen detection methods is complied in the Table 1.
Whereas indirect detection methods works on the plant stress profiling and plant volatile profiling etc. All the methods mentioned in the Table 1. requires high end laboratory and skilled professionals [12-45].
Principle of LAMP technology
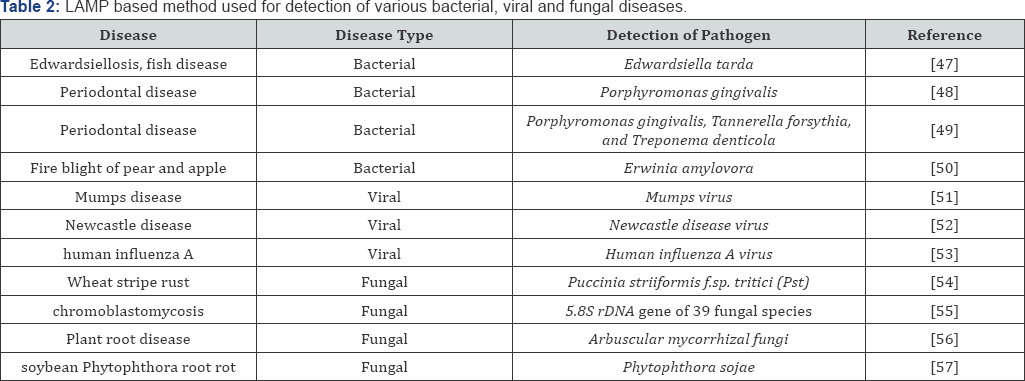
LAMP (Loop-mediated isothermal Amplification) method was reported by Notomy [46]. It is highly specific and sensitive technique to discriminate variations at the single nucleotides polymorphism. It is also a simple, cost effective, rapid and accurate method to be used in the field testing of pathogens. LAMP method does not require specific laboratory set up, it requires only stable heat providing instrument which can be operated through batteries also. The various applications of LAMP technique in the detection of plant pathogenic bacteria, viral and fungal diseases are compiled in the Table 2.
The technique involves the amplification of nucleic acids of plant microbes under isothermal conditions. Primers designed for the assay will amplify the stem-loop DNA structures of microbes with several inverted repeats of the target fragments, so as to detect and differentiate polymorphism among bacteria and fungal pathogens. Technology has been commercially developed by number of diagnostic companies, wherein the concentrations of white precipitates of magnesium pyrophosphate was detected using fluroscent dye like syber green. Fluorescence of syber green dye is the indication of amplification of target nucleic acid fragments of microbes present in human or animal or plant samples [47-56].
Organism specific genes or rRNA region is selected for designing LAMP specific primers. Instead of single primer pairs, LAMP uses 3 or more than three primer pairs, which makes it highly specific for target identification. Several tools and software are available for LAMP specific primer designing but validation is required for all the primer sets. Protocol of LAMP includes crude or pure DNA isolation and isothermal amplification of the DNA using LAMP specific primers followed by visual or spectrophotometeric analysis for detection of change in color as a result of amplification. Few methods uses fluoresce in isothiocyanate (FITC)/biotin for labeling the primers followed by detection based on specially treated lateral flow test strips (Milenia strips, Milenia Biotec Gieben, Germany) [57].
Commercial development of LAMP based devices
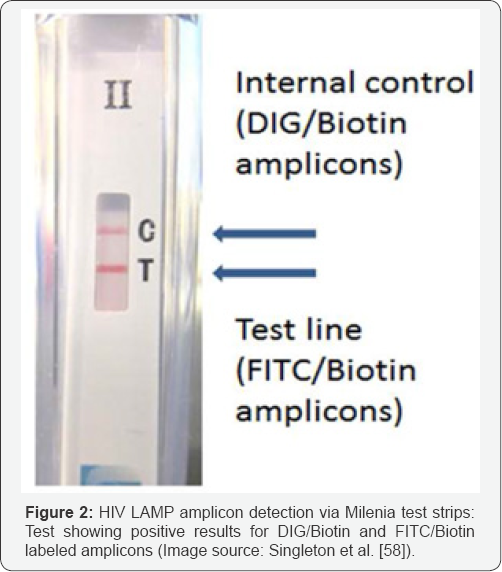
Currently, most researchers are using LAMP method for detecting targeted pathogen by SYBR green or any other fluorescent dye based detection protocol. In this protocol, DNA or cDNA is amplified using target specific LAMP mediated primers. As the amplified products contains loop structures in the positive samples they retains the fluorescent dye and changes their color. This traditional method uses water bath, dry bath or thermal cycler for providing temperature to the reaction. Jered et al. [58] have developed a device named as"Non-instrumented nucleic acid amplification (NINA) platform" (Figure 1) which is an electricity-free platform capable of isothermal amplification and detection of a variety of pathogens. This is a kind of point of care device as it neither requires any specific laboratory/ infrastructure nor electricity. It uses MgFe fuel pouch to activate the heater. MgFe is added to the bottom of the heater and mixed with 5ml of saline which is commercially available as a blow-fill- seal cartridge. Within 12 minutes, temperature increases up to 61.5 °C +/21.5 °C which is desired temperature for performing the assay. They have validated the detection of HIV-1 with a K-actin positive internal amplification control from processed sample to result within 80 minutes time with optimum sensitivity and reproducibility. The heater was used to amplify specific LAMP amplicons in which loop primers used were labeled with hapten to enable capture using antibody binding of FITC followed by visual detection of captured amplicons via streptavidin colloidal gold. LAMP positive reactions were analyzed using Milenia lateral flow test strips (Milenia Biotec Gieben, Germany) (Figure 2). Limit of detection for HIV viral assay performed by this instrument was reported to be 75 copies/reaction or 8,333 viral copies/mL of extracted plasma [58].

Future application
Maize is agriculturally and industrially very important crop for India and world. Its demand is estimated to be doubled by the year of 2050. However, crop productivity is reduced by biotic and abiotic stresses. Both fungal and bacterial pathogens play significant role in the reduction of maize crop yield in India. Early diagnosis of pathogens will decrease the considerable economic loss to Farmers. Xcelris labs is working on developing a LAMP based diagnostic test for detection of Fusarium sps, Pseudomonas sps, and Sclerophthora sps affecting maize crops. After successful development and validation of kit in field, it will help in early stage diagnosis of pathogens in Sorghum, Rice, Wheat, Oats and other Poaceae family members.
Conclusion
LAMP technology is very well studied as a research and investigative project, which has significant impact in the crop management in rural areas of India. Development of LAMP based devices can contribute significantly to crop improvement. Large amount of crop damage in rural India due to delay in the identification and detection of pathogen causing the disease. Therefore, field testing of plant pathogens will help farmers to take crop protection measures, namely, spraying insecticides or pesticides, release of biological predators, fertigation in green house or poly house, early harvesting of crop produce or fruits, proper storage of food grains and so on. LAMP based devices can be made available at Taluka level or village level by bringing awareness in "farmers training centers”.
Conflict of interest and Disclaimer
Authors declare no conflict of interests. Copyrights, trade names and trademarks used in the article belong to the respective companies. Opinions expressed in the article by authors are based on available scientific reviews. Neither Xcelris Labs Limited nor Abellon Group Company recommends or endorses the products or techniques mentioned in the article.
References
- Godfray HC, Beddington JR, Crute IR, Haddad L, Lawrence D, et al. (2010) Food security: the challenge of feeding 9 billion people. Science 327(5967): 812-818.
- Leahy E, Lyons S, Tol RS (2010) An estimate of the number of vegetarians in the world (No. 340). ESRI working paper
- Savary S, Ficke A, Aubertot JN, Hollier C (2012) Crop losses due to diseases and their implications for global food production losses and food security.
- Oerke EC (2006) Crop losses to pests. The Journal of Agri Sci 144(1): 31-43.
- Pimentel D, Zuniga, R, Morrison D (2005) Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecological economics 52(3): 273-288.
- Roberts MJ, Schimmelpfennig DE, Ashley E, Livingston MJ, Ash MS, et al. (2006) The value of plant disease early-warning systems: a case study of USDA's soybean rust coordinated framework (No. 7208). United States Department of Agriculture, Economic Research Service.
- Van der Wolf JM, Van Beckhoven JRCM, Bonants PJM, Schoen CD (2001) New technologies for sensitive and specific routine detection of plant pathogenic bacteria. In Plant pathogenic bacteria Springer Netherlands, pp. 75-77.
- Suga H, Hirayama Y, Morishima M, Suzuki T, Kageyama K, et al. (2013) Development of PCR primers to identify Fusarium oxysporum f. sp. fragariae. Plant disease 97(5): 619-625.
- Bonants P, van Gent-Pelzer MPE, Hooftman R, Cooke D, Guy DC, et al. (2004) A combination of baiting and different PCR formats, including measurement of real-time quantitative fluorescence, for the detection of Phytophthora fragariae in strawberry plants. Eur J Plant Pathol 110(7): 689-702.
- Stöger A, Ruppitsch W (2004) A rapid and sensitive method for the detection of Xanthomonas fragariae, causal agent of angular leafspot disease in strawberry plants. J Microbiol Meth 58(2): 281-284.
- Fang Y, Ramasamy RP (2015) Current and prospective methods for plant disease detection. Biosensors 5(3): 537-561.
- Vandroemme J, Baeyen S, Van Vaerenbergh J, De Vos P, Maes M (2008) Sensitive real-time PCR detection of Xanthomonas fragariae in strawberry plants. Plant Pathol 57: 438-444.
- Ioos R, Laugustin L, Schenck N, Rose S, Husson C, et al. (2006) Usefulness of single copy genes containing introns in Phytophthora for the development of detection tools for the regulated species P. ramorum and P. fragariae. Eur J Plant Pathol 116(2): 171-176.
- Suarez MB, Walsh K, Boonham N, O'Neill T, Pearson S, et al. (2005) Development of real-time PCR (TaqMan®) assays for the detection and quantification of Botrytis cinerea in planta. Plant Physiol Bioch 43(9): 890-899.
- Rigotti S, Gindro K, Richter H, Viret O (2002) Characterization of molecular markers for specific and sensitive detection of Botrytiscinerea Pers.: Fr. in strawberry (Fragariaxananassa Duch.) using PCR. FEMS Microbiol Lett 209: 169-174.
- Debode J, Van Hemelrijck W, Baeyen S, Creemers P, Heungens K, et al. (2009) Quantitative detection and monitoring of Colletotrichum acutatum in strawberry leaves using real-time PCR. Plant Pathol 58(3): 504-514.
- Garrido C, Carbú M, Fernandez-Acero FJ, Boonham N, Colyer A, et al. (2009) Development of protocols for detection of Colletotrichum acutatum and monitoring of strawberry anthracnose using real-time PCR. Plant Pathol 58: 43-51.
- PÞrez-Hernández O, Nam MH, Gleason ML, Kim HG (2008) Development of a nested polymerase chain reaction assay for detection of Colletotrichum acutatum on symptomless strawberry leaves. Plant Dis 92(12): 1655-1661.
- Bilodeau GJ, Koike ST, Uribe P, Martin FN (2012) Development of an assay for rapid detection and quantification of Verticillium dahliae in soil. Phytopathol 102(3): 331-343.
- Bonants P, Weerdt MH, van Gent-Pelzer M, Lacourt I, Cooke D, et al. (1997) Detection and identification of Phytophthora fragariae Hickman by the polymerase chain reaction. Eur J Plant Pathol 103(4): 345-355.
- Kuchta P, Jecz T, Korbin M (2008) The suitability of PCR-based techniques for detecting Verticillium dahliaein strawberry plants and soil. J Fruit Ornamental Plant Res 16: 295-304.
- Pooler MR, Ritchie DF, Hartung JS (1996) Genetic relationships among strains of Xanthomonas fragariae based on random amplified polymorphic DNA PCR, repetitive extragenic palindromic PCR, and enterobacterial repetitive intergenic consensus PCR data and generation of multiplexed PCR primers useful for the identification of this phytopathogen. Appl Environ Microbiol 62(9): 3121-3127.
- Zimmermann C, Hinrichs-Berger J, Moltmann E, Buchenauer H (2004) Nested PCR (polymerase chain reaction) for detection of Xanthomonas fragariae in symptomless strawberry plants. J Plant Dis Protect 111(1): 39-51.
- Roberts PD, Jones JB, Chandler CK, Stall RE, Roberts PD, et al. (1996) Survival of X. fragariae on strawberry in summer nurseries in Florida detected by specific primers and nested PCR. Plant Dis 80(11): 12831288.
- Mahuku GS, Goodwin PH (1997) Presence of Xanthomonas fragariae in symptomless strawberry crowns in Ontario detected using a nested polymerase chain reaction (PCR). Can J Plant Pathol 19(4): 366-370.
- Weller SA, Beresford-Jones NJ, Hall J, Thwaites R, Parkinson N, et al. (2007) Detection of Xanthomonas fragariae and presumptive detection of Xanthomonas arboricola pv. fragariae, from strawberry leaves, by real-time PCR. J Microbiol Methods 70: 379-383.
- Drenth A, Wagels G, Smith B, Sendall B, O'Dwyer C, et al. (2006) Development of a DNA-based method for detection and identification of Phytophthora species. Australas Plant Path 35(2): 147-159.
- Sreenivasaprasad S, Sharada K, Brown AE, Mills PR (1996) PCR-based detection of Colletotrichum acutatum on strawberry. Plant Pathol 45(4): 650-655.
- Zhang S, Goodwin PH (1997) Rapid and sensitive detection of Xanthomonas fragariae by simple alkaline DNA extraction and the polymerase chain reaction. J Phytopathol 145(5-6): 267-270.
- Wullings BA, Beuningen AR van, Janse JD, Akkermans ADL, Van Beuningen AR (1998) Detection of Ralstonia solanacearum, which causes brown rot of potato, by fluorescent in situ hybridization with 23S rRNA-targeted probes. Appl Environ Microbiol 64(11): 4546-4554.
- Caruso P, Gorris MT, Cambra M, Palomo JL, Collar J, et al. (2002) Enrichment double-antibody sandwich indirect enzyme-linkedimmunosorbent assay that uses a specific monoclonal antibody for sensitive detection of Ralstonia solanacearum in asymptomatic potato tubers. Appl Environ Microbiol 68(7): 3634-3638.
- Gorris MT, Cambra M, Llop P, Lopez MM, Lecomte P, et al. (1996) A sensitive and specific detection of Erwinia amylovora based on the ELISA-DASI enrichment method with monoclonal antibodies. Acta Hortic 411: 41-46.
- Dewey F, Marshall G (1996) Production and use of monoclonal antibodies for the detection of fungi. Proceeding of British Crop Protection Council Symposium; Farnham, UK, pp. 18-21.
- Wullings BA, Van Beuningen AR, Janse JD, Akkermans ADL (1998) Detection of Ralstonia solanacearum, which causes brown rot of potato, by fluorescent in situ hybridization with 23S rRNA-targeted probes. Appl Environ Microbiol 64(11): 4546-4554.
- Davey HM, Kell DB (1996) Flow cytometry and cell sorting of heterogeneous microbial populations: the importance of single cell analyses. Microbiol Rev 60(4): 641-696.
- Hijri M (2009) Plant Pathology. The use of Fluorescent in situ hybridisation in plant fungal identification and genotyping. Springer; Berlin, Germany, pp. 131-145.
- Kliot A, Kontsedalov S, Lebedev G, Brumin M, Cathrin PB, et al. (2014) Fluorescence in situ hybridizations (FISH) for the localization of viruses and endosymbiotic bacteria in plant and insect tissues. J Vis Exp 84: e51030.
- Oerke EC, Steiner U, Dehne HW, Lindenthal M (2006) Thermal imaging of cucumber leaves affected by downy mildew and environmental conditions. J Exp Bot 57(9): 2121-2132.
- Hillnhutter C, Mahlein AK, Sikora RA, Oerke EC (2011) Remote sensing to detect plant stress induced by Heterodera schachtii and Rhizoctonia solani in sugar beet fields. Field Crops Res 122(1): 70-77.
- Kuckenberg J, Tartachnyk I, Noga G (2009) Temporal and spatial changes of chlorophyll fluorescence as a basis for early and precise detection of leaf rust and powdery mildew infections in wheat leaves. Precis Agric 10(1): 34-44.
- Delalieux S, van Aardt J, Keulemans W, Schrevens E, Coppin P (2007) Detection of biotic stress (Venturia inaequalis) in apple trees using hyperspectral data: Non-parametric statistical approaches and physiological implications. Eur J Agron 27(1): 130-143.
- Kobayashi T, Kanda E, Kitada K, Ishiguro K, Torigoe Y (2001) Detection of rice panicle blast with multispectral radiometer and the potential of using airborne multispectral scanners. Phytopathology 91(3): 316323.
- Zhang M, Qin Z, Liu X, Ustin SL (2003) Detection of stress in tomatoes induced by late blight disease in California, USA, using hyperspectral remote sensing. Int J Appl Earth Observ Geoinf 4(4): 295-310.
- Fang Y, Umasankar Y, Ramasamy RP (2014) Electrochemical detection of p-ethylguaiacol, a fungi infected fruit volatile using metal oxide nanoparticles. Analyst 139: 3804-3810.
- Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, et al. (2000) Loop-mediated isothermal amplification of DNA. Nucleic acids research 28(12): e63-e63.
- Savan R, Igarashi A, Matsuoka S, Sakai M (2004) Sensitive and rapid detection of edwardsiellosis in fish by a loop-mediated isothermal amplification method. Appl Environ Microbiol 70(1): 621-624.
- Maeda H, Kokeguchi S, Fujimoto C, Tanimoto I, Yoshizumi W, et al. (2005) Detection of periodontal pathogen Porphyromonas gingivalis by loop-mediated isothermal amplification method. FEMS Immunology & Medical Microbiology 43(2): 233-239.
- Yoshida A, Nagashima S, Ansai T, Tachibana M, Kato H, et al. (2005) Loop-mediated isothermal amplification method for rapid detection ofthe periodontopathic bacteria Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola. J Clin Microbiol 43(5): 2418-2424.
- Temple TN, Johnson KB (2010) Evaluation of Loop-Mediated Isothermal Amplification for Rapid Detection of Erwinia amylovora on Pear and Apple Fruit Flowers. Plant Dis 95(4): 423-430.
- Okafuji T, Yoshida N, Fujino M, Motegi Y, Ihara T, et al. (2005) Rapid diagnostic method for detection of mumps virus genome by loop-mediated isothermal amplification. Journal of clinical microbiology 43(4): 1625-1631.
- Pham HM, Nakajima C, Ohashi K, Onuma M (2005) Loop-mediated isothermal amplification for rapid detection of Newcastle disease virus. Journal of clinical microbiology 43(4): 1646-1650.
- Poon LL, Leung CS, Chan KH, Lee JH, Yuen KY (2005) Detection of human influenza A viruses by loop-mediated isothermal amplification. J Clin Microbiol 43(1): 427-430.
- Huang C, Sun Z, Yan J, Luo Y, Wang H, et al. (2011) Rapid and Precise Detection of Latent Infections of Wheat Stripe Rust in WheatLeaves using Loop-Mediated Isothermal Amplification. Journal of Phytopathology 159(7-8): 582-584.
- Sun J, Najafzadeh MJ, Vicente V, Xi L, de Hoog GS (2010) Rapid detection of pathogenic fungi using loop-mediated isothermal amplification, exemplified by Fonsecaea agents of chromoblastomycosis. J Microbiol Methods 80(1): 19-24.
- Gadkar V, Rillig, MC (2008) Evaluation of loop-mediated isothermal amplification (LAMP) to rapidly detect arbuscular mycorrhizal fungi. Soil Biology and Biochemistry 40(2): 540-543.
- Dai TT, Lu CC, Lu J, Dong S, Ye W, et al. (2012) Development of a loopmediated isothermal amplification assay for detection of Phytophthora sojae. FEMS microbiology letters 334(1): 27-34.
- Singleton J, Osborn JL, Lillis L, Hawkins K, Guelig D, et al. (2014) Electricity-free amplification and detection for molecular point-of-care diagnosis of HIV-1. PloS one 9(11): e113693.






























