Scanning Electron Microscopic Analysis of Glycated Histone H2B
Abdul Rouf Mir*
Department of Biotechnology, University of Kashmir Jammu and Kashmir, India
Submission: May 01, 2017; Published: May 24, 2017
*Corresponding author: Abdul Rouf Mir, Department of Biotechnology, Govt. Degree College Baramulla, University of Kashmir, India, Email: roufonline@gmail.com
How to cite this article: Abdul R M. Scanning Electron Microscopic Analysis of Glycated Histone H2B. Curr Trends Biomedical Eng & Biosci. 2017; 4(2): 555631. DOI: 10.19080/CTBEB.2017.04.555631.
Abstract
Glycation is a widely reported modification of proteins. It leads to the compromise in the structural integrity of the proteins and have been widely implicated in pathological problems. While a large number of protein structures have been analysed for post glycation effects, no work reports changes in histone H2B upon non enzymatic glycation. The work presents the scanning electron micrographic study on the structure of histone H2B derived from calf thymus after its modification by an oxo-aldehyde named methylgyoxal. The results reveal a complete different look of H2B post modification under SEM, and the images point towards the amorphous aggregate adduct generation. While, many protein modifications may have little significance, the modifications of H2B can have severe impact on the gene expression and epigenetics as it is a part of core histone octamer. The work needs to be taken forward for further analysis.
Introduction
Glycation is a multi-step series of events wherein nucleophilic part of a proteins reacts with the sugars or their aldehyde and ketone derivatives leading to the generation of irreversible cross-linked products, described as advanced glycation end products (AGEs) [1]. The research has revealed that a-dicarbonyls or oxoaldehydes are the most potent glycating agents that damage the structural integrity of proteins [2]. Glycation and its associated carbonyl and oxidative stress have clinical implications in multiple pathological conditions including diabetes and cancer [3-5]. Giovannucci et al. [6] have pointed towards a possibility wherein hyperglycemia in diabetes conditions may confer a growth advantage to the tumors and have also suggested not-to overlook even glucose as a potentially relevant mediator. It may be noted that hyperglycemia leads to the accumulation of AGEs. In the context, the glycation induced modification of proteins and the structural and conformational changes thereof have emerged as an important field of study. In our earlier works, we have established the role of glycation in histone H1 and H2A modification [5,7]. In this study we present the scanning electron microscopic analysis of glycation of histone H2B. Histone H2B is a14 kDa protein that consists of 126 amino acid residues with abundance of lysine and arginine residues. The later make the protein susceptible to glycation. Its N-terminal tails are the most accessible regions of its peptide as they protrude from the nucleosome and they have been reported to be prone to various post-translational modifications (PTMs). H2B is a basic protein that is part of the histone octamer and is found associated with DNA in cell nuclei of eukaryotic cells [8].
H2B glycation
The concentration of H2B was ascertained by using molar absorption coefficient (280 nm) of 6400 M-1 cm-1 and the molecular weight of 13774 Dalton for calf thymus H2B. Earlier published protocol was adopted wherein H2B (50μM) was glycated by 7.5mM of methylglyoxal in phosphate buffer saline (10 mM sodium phosphate buffer, pH 7.4 containing 150mM NaCl) and incubated overnight [5].
Scanning electron microscopy (SEM)
The unmodified and the glycated samples of H2B protein were subjected to scanning electron microscopic analysis to obtain the alterations in its structural architecture. Samples were air dries and adsorbed upon the cellulose ultra filtration membrane that was coated with gold. The sample was mounted on a carbon tape that had coated stainless steel grids working at 15kV. The instrument employed was a JSM-6510LV (JEOL JAPAN) scanning electron microscope running. The detailed protocol has been presented earlier [9].
Results
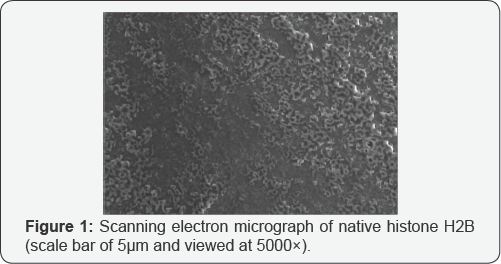
The images obtained under SEM have been provided as Figure 1 for native H2B and Figure 2 for glycated H2B. The results reveal a dramatic change in the structural integrity of the protein.

Discussion
Glycation induced structural modifications have been presented by wide number of workers [1-14]. However, there is no study on the SEM studies of histone H2B in the published literature. Our experimental analysis of H2B glycation reveals severe damage of protein integrity as is evident from the morphological featuring of the glycated H2B. Visibly, the native protein appears to show no aggregation. Incubation with methylglyoxal has changed altogether in shape and its presents an amorphous type aggregate adduct. The formation of in proteins upon sugar mediated modifications can be traced from earlier papers as well [9-11]. Aggregation of glycated proteins has been observed with varied shapes and sizes and many have reported clustered granular precipitates and large branched chain shapes in the modified proteins as well [12]. The aggregation of proteins has been attributed with cytotoxic implications in the earlier literature [10-13]. This study and its related works confirm the diversity of aggregates formed in proteins upon glycation. To conclude, it may be argued that histone glycation is a well reported phenomenon and this study extends the work to H2B histone, wherein glycation led to the generation of amorphous aggregates [14-15].
References
- Anguizola J, Matsuda R, Barnaby OS, Hoy KS, Wa C, et al. (2013) Glycation of human serum albumin. Clin Chim Acta 425: 64-76.
- Groener JB, Oikonomou D, Cheko R, Kender Z, Zemva J, et al. (2017) Methylglyoxal and Advanced Glycation End Products in Patients with Diabetes - What We Know so Far and the Missing Links. Exp Clin Endocrinol Diabetes.
- Singh R, Barden A, Mori T, Beilin L (2014) Advanced glycation end- products: a review. Diabetologia 44(2): 129-146.
- Chuah YK, Basir R, Talib H, Tie TH, Nordin N (2013) Receptor for advanced glycation end products and its involvement in inflammatory diseases. Int J Inflam, 403460.
- Mir AR, Uddin M, Khan F, Alam K, Ali A (2015) Dicarbonyl Induced Structural Perturbations Make Histone H1 Highly Immunogenic and Generate an Auto-Immune Response in Cancer. PLoS One 10(8): e0136197.
- Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, et al. (2010) Diabetes and Cancer: A consensus report. Diabetes Care 33(7): 1674-1685.
- Mir AR, Uddin M, Alam K, Ali A (2014) Methylglyoxal mediated conformational changes in histone H2A-generation of carboxyethylated advanced glycation end products. Int J Biol Macromol 69: 260-266.
- Kahl G (2015) The Dictionary of Genomics, Transcriptomics and Proteomics, 4 Volume Set, 5th Edition.
- Iram A, Alam T, Khan JM, Khan TA, Khan RH, Naeem A (2013) Molten globule of hemoglobin proceeds into aggregates and advanced glycated end products. PLoS ONE 8(8): e72075.
- Ishimaru D, Andrade LR, Teixeira LSP, Quesado PA, Maiolino LM, et al. (2003) Fibrillar aggregates of the tumor suppressor p53 core domain. Biochemistry 42(30): 9022-9027.
- Bouma B, Kroon-Batenburg LM, Wu YP, Brúnjes B, Posthuma G, et al. (2003) Glycation induces formation of amyloid cross-beta structure in albumin. J Biol Chem 278(43): 41810-41819.
- Mir AR, Moinuddin, Habib S, Khan F, Alam K, et al. (2016) Structural changes in histone H2A by methylglyoxal generate highly immunogenic amorphous aggregates with implications in auto-immune response in cancer. Glycobiology (2): 129-141
- Bucciantini M, Giannoni E, Chiti F, Baroni F, Formigli L Zurdo J, et al. (2002) Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature 416: 507-511.
- Pashikanti S, Boissonneault GA, Cervantes-Laurean D (2011) Ex vivo detection of histone H1 modified with advanced glycation end products. Free Radic Biol Med 50(10): 1410-1416.
- Mir AR, Moinuddin (2015) Glycoxidation of histone proteins in autoimmune disorders. Clin Chim Acta 450: 25-30.






























