Mini-Review on Electro-coagulation for Wastewater Treatment
Swachwa Dey1,2 and Kyu Taek Cho1,2*
1Mechanical Engineering, Northern Illinois University, USA
2Electrochemical Thermal Energy Laboratory, Northern Illinois University, USA
Submission: February 01, 2024; Published: February 22, 2024
*Corresponding Author: Kyu Taek Cho, 1Mechanical Engineering, Electrochemical Thermal Energy Laboratory, Northern Illinois University, Dekalb, IL, USA
How to cite this article: Swachwa Dey and Kyu Taek Cho*. Mini-Review on Electro-coagulation for Wastewater Treatment. Civil Eng Res J. 2024; 14(3): : 555889. DOI 10.19080/CERJ.2024.14.555889
Abstract
The electro-coagulation (EC) system has been investigated as a promising solution for wastewater treatment. Due to its excellent benefits such as environmentally friendly effects and cost-effective operation, it has garnered significant interest recently, resulting in the publication of hundreds of research articles. These articles delve not only into fundamental studies based on mathematical modeling and experiments but also into its applications. This study reviews the EC system for its fundamental working principles and key features, recent advancements, and its status towards industrial applications. Additionally, it explores how EC treatment can be applied to various types of pollutants, both organic and inorganic. System integration of EC with other treatment subsystems is a promising method to complement EC function, leading to a much more efficient method for industrial application. This mini-review will provide key information on the EC system and the status of research, development, and application of EC systems.
Keywords: Electrocoagulation; Chemical treatment; Wastewater treatment; Flotation, and Flocculation; Faraday’s law
Abbreviations: EC: Electro Coagulation; CC: Chemical Coagulation; EO: Electro Oxidation; MMO: Mixed Metal Oxide; NOM: Natural Organic Matter; COD: Chemical Oxygen Demand; SS: Suspended Solid; BOD: Biological Oxygen Demand
Introduction
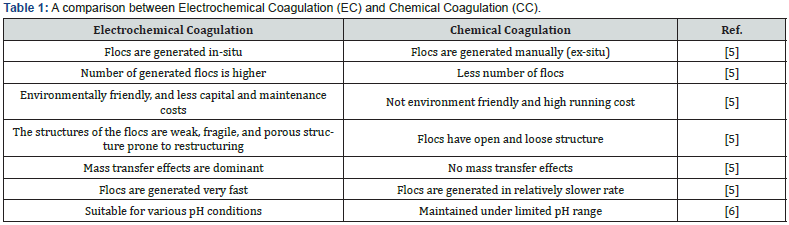
There has long been a desire to find sustainable solutions for efficient wastewater management. Electrocoagulation (EC) is considered one of the most promising options due to its economic and environmentally friendly features [1]. In recent years, extensive research has been conducted on EC to gather information about its environmental effects and its potential as a solution to water scarcity [2]. Between 2005 and 2020, there were 7903 research articles on this promising system. 46% of these publications focused on the system itself since 1922, with 20% published from 2015 to 2020 [3]. EC systems gained significant attention due to research uncovering their features, including relatively simple operating procedures, production of colorless and odorless treated water, and minimal sludge production without complex secondary chemical reactions [3]. Unlike chemical treatment systems, EC systems do not require additional chemical coagulants in the reactors. Instead, they can generate sufficient in-situ coagulant through electrochemical and chemical reactions. Consequently, EC systems have a lower risk of secondary contamination compared to chemical coagulation (CC) systems. A brief comparison between EC and CC systems is provided in Table 1. This wastewater treatment process includes not only electrochemical and chemical reactions but also coagulation, flotation, and flocculation, leading to highly efficient removal [4], thus the industrial applications of EC procedures have grown exponentially recently [3].
Working Principles
EC systems typically utilize Al and Fe sacrificial electrodes. These materials are low-cost, readily available, and importantly, the hydroxides generated from them exhibit high capacities for adsorbing soluble contaminants [7]. When current is applied in an EC system, metal ions (Fe2+ or Al3+) from the sacrificial anode and OH- ions from the cathode are released through electrochemical reactions as illustrated in Figure 1. These ions then move towards the bulk solution and participate in several stages of hydrolysis via chemical reactions, generating flocs. The contaminants in the solution are then absorbed on the surface of these insoluble hydroxides or flocs [8,9]. Floc generation in the EC cell can be calculated by Faraday’s law, which establishes the relationship between applied current density and the number of ions generated from the sacrificial metallic electrodes [10].

Where W is floc produced or electrode dissolved; I is the applied current; t is the time duration of current applied to the EC cell; Mw is the molecular weight of the electrode; Z is the number of ions involved in oxidation-reduction reaction and F is the Faraday’s constant whose value is 96500 C/mol of electrons. The initial pH condition of the solution plays a key role in floc production as well as the generation of interim species during the intermediate monomeric and polymeric reactions [3]. Several other factors have also been extensively researched for optimizing the performance of the EC system, including cell gap, electrode materials, surface area, operating time, flow velocity, pollutants’ compositions etc. [11].
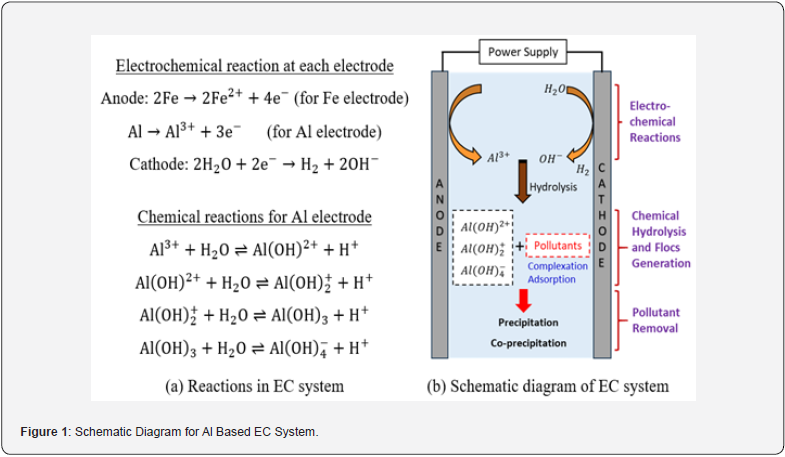
Applications
The EC system has been investigated for treating both inorganic and organic pollutants. For inorganic anions and oxyanions such as fluoride ion (F-), chromate ion (CrO4 2-) and arsenate (HAsO4 2- ), Fe and Al-based hydroxides have provided good results for wastewater treatment [12,13]. It has been observed that F- ions form complexes with Al-based hydroxides Al (OH)3-xFx while other elements like arsenate, sulfates, and phosphates are adsorbed onto the flocs [14,15]. In chromate (Cr) removal, pH conditions, applied current, and electrode material are found to be key factors in EC process. Cr (III) is treated through precipitation with Albased hydroxide flocs as Cr (OH)3 [16-18]. High current density is seen to be helpful for reducing Cr (VI) to Cr (III), whereas low current is found to be more effective for Cr (VI) with Fe-based EC systems [19]. One of the most common water contaminants is phosphate generating eutrophication, often found in ponds and lakes, with numerous uses in the agricultural sector. Through EC treatment, phosphate is extracted from water by adsorption and precipitation [20,21]. Some researchers have conducted investigations by combining electro-oxidation (EO) and EC to treat phosphate, using mixed metal oxide (MMO) electrodes. The combined system delivered 75% efficiency, significantly higher than systems that did not utilize MMO electrodes (less than 23%) and chemical coagulation (less than 5%) [5]. A recent investigation has been conducted to study phosphate removal under very low applied current density (0.04 A/m2) by utilizing graphite felt electrode with a comparatively large surface area (438±20 m2/m2). This study found that phosphate was removed by electrochemically induced calcium phosphate precipitation without significant presence of adsorption [22]. Heavy metals such as cadmium, cyanide, chromium, zinc, lead, mercury, arsenic are also treated through EC systems. These heavy metals are removed through mechanisms like surface complexation, coprecipitation, and adsorption [5,23,24].
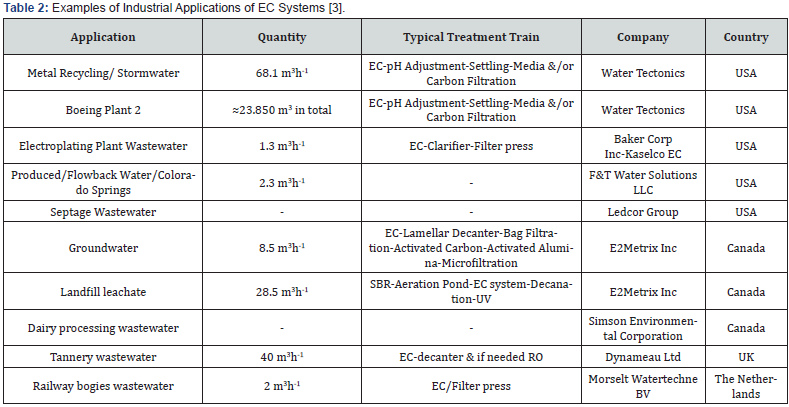
EC technology has also been studied for the treatment of organic pollutants. A wide range of organic pollutants such as natural organic matter (NOM), chemical oxygen demand (COD), suspended solid (SS), biological oxygen demand (BOD), color, oil, and other organic industrial contaminants from tannery, textile, paper mills etc., have been investigated for treatment using EC technique. The key mechanisms for treating these pollutants include direct/indirect oxidation, charge neutralization, entrapment, adsorption and/or combinations of these techniques [5,25]. Currently, there are many applications available for utilizing EC systems in the industrial sector. A notable example is a stormwater recycling facility in Oregon. In that facility, methods like EC, pH adjustment, settling, and media filtration are applied to treat chromium, copper, iron, lead, manganese, and zinc, achieving respective efficiencies of 88%, 91%, 98%, 98%, 91%, and 95% [3]. Water Tectonics Inc., WA holds a patent for an EC system designed for cartridge-type reactors and rod-shaped electrodes [3]. Some successful EC applications in the industrial sector are listed in Table 2.
Conclusion and Future Direction
The scarcity of pure drinking water is already a prevalent issue in many areas of the world. This electrocoagulation (EC) technique has the potential to become a game-changer in the water purification sector, as it offers both economic and environmentally friendly characteristics, which are rare to find together in water purification methods. Moreover, managing EC requirements and consequential effects is relatively simple. Due to its benefits, the application of EC in industries has significantly increased recently.
A significant amount of research has been conducted on the EC system to elucidate underlying physics and key control parameters. However, there are still many unknowns influencing the efficiency of the EC system that require further investigation through comprehensive research combining modeling and experimentation. Additionally, much of the research is focused on specific contaminants, thus necessitating more versatile studies and solutions for widespread industrial adoption.
References
- Merzouk B, Yakoubi M, Zongo I, Leclerc J-P, Paternotte G, et al. (2011) Effect of modification of textile wastewater composition on electrocoagulation efficiency. Desalination 275: 181-186.
- Jing G, Ren S, Gao Y, Sun W, Gao Z (2020) Electrocoagulation: A Promising Method to Treat and Reuse Mineral Processing Wastewater with High COD. Water 12: 595.
- Magnisali E, Yan Q, Vayenas DV (2022) Electrocoagulation as a revived wastewater treatment method‐practical approaches: a review. J Chem Technol Biotechnol 97(1): 9-25.
- Moussa DT, El-Naas MH, Nasser M, Al-Marri MJ (2017) A comprehensive review of electrocoagulation for water treatment: Potentials and challenges. J Environ Manage 186: 24-41.
- Tegladza ID, Xu Q, Xu K, Lv G, Lu J (2021) Electrocoagulation processes: A general review about role of electro-generated flocs in pollutant removal. Process Saf Environ Prot 146: 169-189.
- Harif T, Khai M, Adin A (2012) Electrocoagulation versus chemical coagulation: Coagulation/flocculation mechanisms and resulting floc characteristics. Water Res 46: 3177-3188.
- Mollah MYA, Gomes JAG, Das KK, Cocke DL (2010) Electrochemical treatment of Orange II dye solution-Use of aluminum sacrificial electrodes and floc characterization. J Hazard Mater 174: 851-858.
- Lu J, Li Y, Yin M, Ma X, Lin S (2015) Removing heavy metal ions with continuous aluminum electrocoagulation: A study on back mixing and utilization rate of electro-generated Al ions. Chem Eng J 267: 86-92.
- Lu J, Wang Z, Ma X, Tang Q, Li Y (2017) Modeling of the electrocoagulation process: A study on the mass transfer of electrolysis and hydrolysis products. Chem Eng Sci 165: 165-176.
- Mollah M, Morkovsky P, Gomes J, Kesmez M, Parga JC (2004) Fundamentals, present and future of electrocoagulation. J Harzardous Mater 114(1-3): 199-210.
- Dey S, Adejinle A, Cho KT (2023) Modeling Study of Aluminum-Based Electrocoagulation System for Wastewater Treatment. J Environ Eng 150(2): 04023099.
- Li M, Liu J, Xu Y, Qian G (2016) Phosphate adsorption on metal oxides and metal hydroxides: A comparative review. Environ Rev 24(3): 319-332.
- Seftel EM, Ciocarlan RG, Michielsen B, Meynen V, Mullens S, et al. (2018) Insights into phosphate adsorption behavior on structurally modified ZnAl layered double hydroxides. Appl Clay Sci 165: 234-246.
- Emamjomeh MM, Sivakumar M, Varyani AS (2011) Analysis and the understanding of fluoride removal mechanisms by an electrocoagulation/flotation (ECF) process. Desalination 275: 102-116.
- Lu J, Tang Q, Wang Z-R, Xu C, Lin S-L (2016) A study on continuous and batch electrocoagulation process for fluoride removal. Desalination Water Treat 57: 28417-28425.
- Golder AK, Samanta AN, Ray S (2006) Anionic reactive dye removal from aqueous solution using a new adsorbent—Sludge generated in removal of heavy metal by electrocoagulation. Chem Eng J 122: 107-115.
- Golder A, Samanta A, Ray S (2007) Removal of Cr3+ by electrocoagulation with multiple electrodes: Bipolar and monopolar configurations. J Hazard Mater 141(3): 653-661.
- Golder AK, Chanda AK, Samanta AN, Ray S (2007) Removal of Cr (VI) from Aqueous Solution: Electrocoagulation vs Chemical Coagulation. Sep Sci Technol 42(10): 2177-2193.
- Heidmann I, Calmano W (2008) Removal of Cr (VI) from model wastewaters by electrocoagulation with Fe electrodes. Sep Purif Technol 61(1): 15-21.
- Hashim KS, Al Khaddar R, Jasim N, Shaw A, Phipps D, et al. (2019) Electrocoagulation as a green technology for phosphate removal from river water. Sep Purif Technol 210: 135-144.
- Tian Y, He W, Liang D, Yang W, Logan BE, Ren N (2018) Effective phosphate removal for advanced water treatment using low energy, migration electric–field assisted electrocoagulation. Water Res 138: 129-136.
- Lei Y, Geraets E, Saakes M, Van Der Weijden RD, Buisman CJN (2020) Electrochemical removal of phosphate in the presence of calcium at low current density: Precipitation or adsorption? Water Res 169: 115207.
- Fu F, Wang Q (2011) Removal of heavy metal ions from wastewaters: A review. J Environ Manage 92(3): 407-418.
- Lu J, Wang Z-R, Liu Y-L, Tang Q (2016) Removal of Cr ions from aqueous solution using batch electrocoagulation: Cr removal mechanism and utilization rate of in situ generated metal ions. Process Saf Environ Prot 104: 436-443.
- Arturi TS, Seijas CJ, Bianchi GL (2019) A comparative study on the treatment of gelatin production plant wastewater using electrocoagulation and chemical coagulation. Heliyon 5(5): e01738.






























