Environmental Impact Assessment of Waste Dumpsite using Integrated Geochemical and Physico-Chemical Approach: A Case Study of Ilokun Waste Dumpsite, Ado - Ekiti, Southern Nigeria
Emmanuel Olagunju, Olabode Badmus, Funmilola Ogunlana, Michael Babalola
1Department of Mathematical and Physical Sciences, Afe Babalola University, Ado-Ekiti, Nigeria
2Department of Physics, Ekiti State University, Ado-Ekiti, Nigeria
Submission: December 18, 2017; Published: March 21, 2018
*Corresponding author: Olagunju Emmanuel, Department of Mathematical and Physical Sciences, Afe Babalola University, Ado-Ekiti Nigeria, E-mail: emmanueloo@abuad.edu.ng
How to cite this article:Emmanuel Olagunju, Olabode Badmus, Funmilola Ogunlana, Michael Babalola. Environmental Impact Assessment of Waste Dumpsite using Integrated Geochemical and Physico-Chemical Approach: A Case Study of Ilokun Waste Dumpsite, Ado - Ekiti, Southern Nigeria. Civil Eng Res J. 2018; 4(2): 555631. DOI: 10.19080/CERJ.2018.04.555631
Abstract
Environmental impact assessment of area around Ilokun waste dumpsite was carried-out in other to evaluate the possible impact of the waste dumpsite on soil and ground water in and around Ilokun environs. Thirteen (13) soil samples were collected using a hand auger. The soil samples were collected at horizon B of the soil subsurface. The soil samples were designated S1 to S13. Samples S1 to S12 were collected in and around the waste dumpsite while sample S13 was collected far away to serve as the control point. Seven (7) water samples were collected in and around the waste dumpsite. Five (5) water samples from hand dug well (HDW) and two (2) water samples were collected from boreholes (BH).The concentration of cations such as K, As, Zn, Rb, Sr, Zr, P, Ni, Th and Y which ranges from 542 to 1065ppm, 70 to 1131ppm, 166 to 1257ppm,113 to 1094ppm, 251 to 714ppm, 1500 to 12914ppm, 24 to 161352ppm, 500 to 2444ppm, 441 to 1034ppm and 42ppm to 219ppm respectively. Which were found to exceed the control point, which has a concentration of488ppm, 25ppm, 125ppm, 63ppm, 100ppm, 1471ppm, 0ppm, 424ppm and 421ppm and 0ppm respectively. The concentration of cations such as Fe, Mg, Mn and Pb of concentration range are relatively high in water samples and are found to 0.177 to 0.503ppm, 3.520 to 4.566ppm, 0.128 to 0.313ppm and 0.001 to 0.031ppm respectively. They are found to exceed the Nigerian standard for quality drinking water [1] and the World health organization [2] standards. The alkalinity concentration in water in the area ranges 120.0 to 232.0mg CaCO3/l which exceeds the maximum required standard for drinking water. The result obtained also show an exceptionally high values of toxic metals such as Arsenic and zinc in the soil in the study area indicate that the soil in the area is not suitable for agricultural activities. While high concentration values of metals such as iron and lead in some wells in the study area showed that the ground water in most of the boreholes and hand-dug wells sampled is not suitable for human consumption. This gives course for concern, since the people living in this area depend on food from agricultural activities in the area and water supply from the hand-dugwells and boreholes for various domestic purposes. There is, therefore the need to carryout further geophysical modeling methods to ascertain the full extent of contamination in the area in other to alert the local authority in the area of this dangerous trend, so that an alternative arrangement can be made to provide food and domestic water for residents of the area.
Keywords:Waste dumpsite; Soil; Groundwater; Leachate; Anions; Cations; Ilokun
Abbrevations:GPS: Global Positioning Systems; CERD: Centre for Energy Research and Development; XRF: X-Ray Fluorescence; AAS: Atomic Absorption Spectrometry; HDW: Hand Dug Wells; OAU: Obafemi Awolowo University; TDS: Total Dissolved solids; EC: Electrical Conductivity; K: Potassium; Ca: calcium; Mg: Magnesium; Al: Aluminum; Ti: Titanium; Mn: Manganese; Fe: Iron; As: Arsenic; Zn: Zinc; Rb: Rubidium; Sr: Strontium; Zr: Zirconium; Nb: Niobium; Ce: Cerium; P: Phosphorus; Pb: Lead; Cd: Cadmium; Ni: Nickel; Cu: Copper; Cr: Chromium; Th: Thorium; Uranium; Ba: Barium; Y: Yttrium; OAU: Obafemi Awolowo University
Introduction
Wastes are the unwanted or useless solid materials generated from the combined residential, industrial, and commercial activities of a given area. Solid wastes could be categorized according to its origin (domestic, industrial, commercial, construction or institutional); according to its contents (organic material, glass, metal, plastic paper, etc.) or according to its hazard potential (toxic, non-toxin, flammable, radioactive, inflections, etc) [3]. Municipal Solid Waste (MSW) are described as domestic as well as commercial waste that account for a relatively small part of the total solid waste stream in developing countries [4]. A municipal solid waste dumpsite is not a benign repository of discarded material; it is a biochemically active unit where toxic substances are leached or created from combinations of nontoxic precursors and gradually released into the surrounding environment over a period of decades [5].
Over the last couple of decades there has been an increase in the effect of environmental pollution on the health of the populace due to the improper disposal of waste. Improper waste disposal has become a major environmental issue affecting not only the health of the general public (health hazards) but also the surrounding environment (environmental hazards). The most prominent result of health hazards is the wide spread of communicable diseases like cholera. Hoornweg [6] posited that "The complexities of waste which modern civilization produce is directly related to the living standards, socio-economic and cultural attributes of that particular environment". He also asserted that solid waste streams could be characterized by their sources, type of waste (solid, liquid, or gaseous states) produced as well as generation rate and composition. He classified wastes into eight, namely; residential, industrial, commercial, institutional, constructional, demolition, municipal services, process and agriculture [7]. Disposal of solid waste is essential in the management of solid waste; this is required to avoid environmental pollution and health problems. However most solid waste disposal sites are found most commonly along the outskirts of urban areas where there are water bodies and scattered settlements [8]. Inappropriate disposal of solid waste lead to contamination of surface and ground water through leachate, soil contamination through direct waste contact, air pollution by burning of wastes, spreading of diseases by different vectors like birds, insects and rodents, or uncontrolled re-lease of methane by anaerobic decomposition of waste [9]. Dumpsites are areas where waste materials are disposed and are viewed as the oldest form of waste treatment. Historically, dumpsites have been the most common method of unorganized waste disposal in many places around the world. Most dumpsites are located within the vicinity of living communities and wetlands [10]. Local dumpsites are often not lined nor basement prepared for selective absorption of toxic substances. Therefore it is prone to release of pollutant to nearby water and to air through leachates and dumpsite gases respectively. Wet waste decomposes and releases a bad odour. The bad odour affects the people living around such dumpsites, which show that dumpsites have serious effect on people around its environment. Also dumpsites found close to residential areas are found to be suitable sites for feeding for animals (dogs, pets) which could carry diseases with them to homesteads around.
Nigeria today being the most populous country in Africa with a continuous growing population of above150 million people. The growth of population in urban areas is been characterized by continuous increase in the amounts of waste generated thus the need arises for an efficient waste disposal system.
The Ilokun waste dumpsite under investigation is located in Ado-Ekiti southwestern Nigeria, it has been in active existence for the last 20 years. The dumpsite host various types of waste such as garbage, paper, plastic, glass, metal scape etc. Some of these waste are inhomogeneous materials which are largely non- biodegradable and has been compacted over the years which has allow long time interaction between the dumpsite materials, the soil and the subsurface geological unit. Waste deposited into dumpsite undergoes oxidation, corrosion of metallic components and decomposition of organic matter resulting in the generation and release of leachate which can impact the soil surface and groundwater resources and thereby affecting the portability of underground water [11].
However, they are possible indications that the leachate generated from the dumpsite may have an impact on the immediate environment [12]. To determine the extent of possible contamination of surface, subsurface soil and groundwater in the area, physico-chemical and geochemical investigations were carried-out in the area.
Raman N and Warayanan [13] investigated the impact solid waste effort in groundwater and soil quality near to pallavarem solid waste landfill site in Chennai, India. Other related literatures such as Byoung- Young et al. [14], Kassenga and Mbuligwe [15], Nartey et al. [16], Georgeet al. [17], Bayode and Adeniyi [11] and Olagunju et al. [18].
Description of the study area
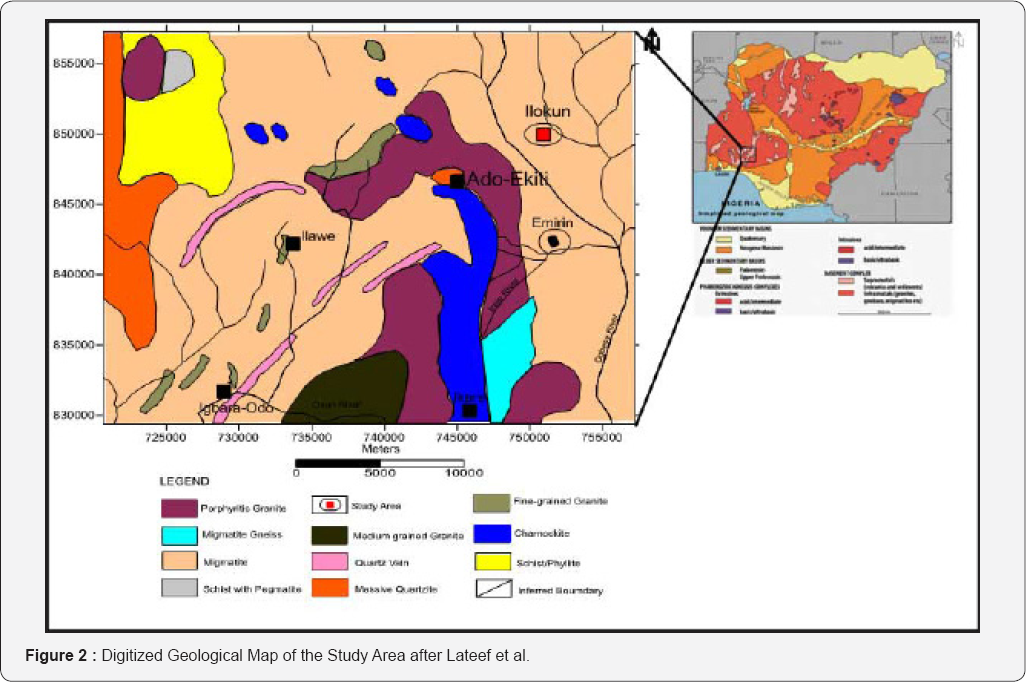
Ilokun, the study area is located in Ado-Ekiti, Local Government area of Ekiti state. The study area lies between the geographic coordinates (UTM) of latitudes 850200 to 850800 and Longitude 749300 and 749800, (Figure 1). The topographic evaluation around the dumpsite ranges from 337.4m to 405.2m above mean sea level and generally slopes gently from the north western part towards south eastern part. The investigated dumpsite covers an area extent of about 246,980.76m2 (24.7 hectares) as shown in Figure 1. The area has a climate characterized by two seasons: the wet season and the dry season. The wet season starts from around march and ends in October within the average rainfall of 1600mm to 1800mm. while the dry seasons starts around November and ends in march with an average maximum temperature of about 30 °C [19].
Geology and hydrogeological setting
The study area is underlain by the Precambrian basement complex of south western Nigeria. The rock type of the study area was observed to be migmatite (Figure 2). Groundwater is found in the weathered and fractured basement column. The weathered layer in the study area is generally thin due to shallow depth of the bedrock [19]. Based on this, subsurface structural discontinuities (shear zones, fractures and joints) are targeted for productive boreholes in the study area [20].

Method of Study
Soil sample methods
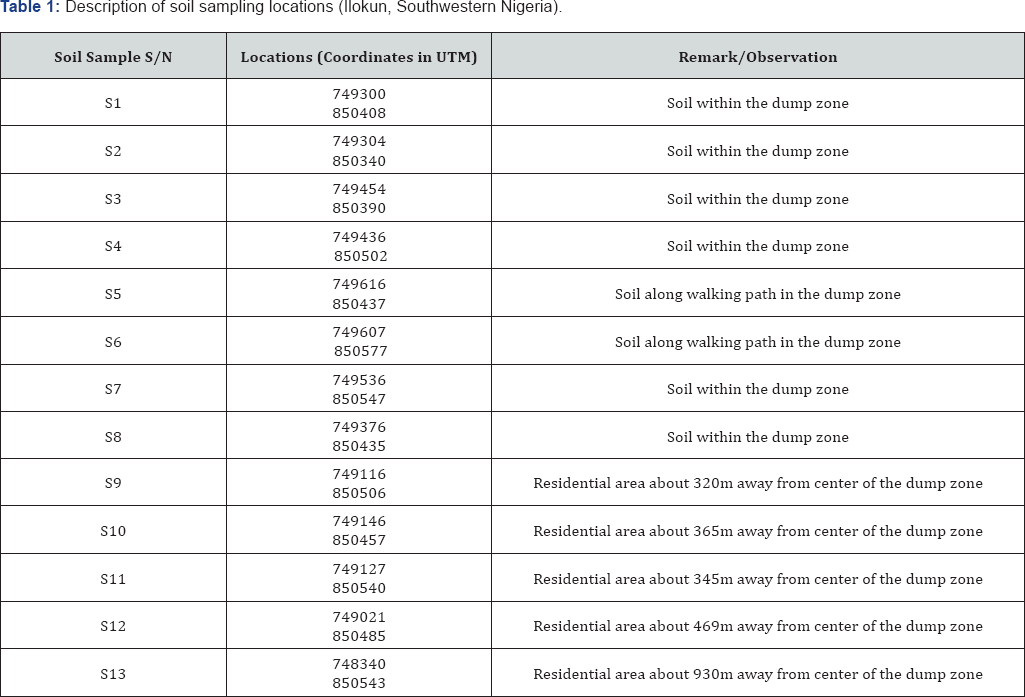
A total of thirteen (13) soil samples from in and outside the waste dumpsite were collected for this study. Using the hand auger at a depth of 50cm below the ground. Figure 3 shows the sampling points in the study area. The soil samples were collected at random interval from the center to the outskirt of the study area. The sampling was done on August 2017 and the Global Positioning Systems (GPS) readings of all the sampled soil was taken for accurate location (Table 1). The samples were designated as S1 to S13 which was taken at random interval.S1 to S8 was taken within the dump zone, S9 to S12 was taken within the residential area away from the dump zone while sample S13 serves as the control point as shown in Figure 3. This is done in order to effective estimate the possible extent of contamination of the soil by the waste dumpsite. The analysis was carried out at the Centre for Energy Research and Development (CERD), Obafemi Awolowo University, Ile - Ife Nigeria, using Atomic Absorption Spectrometry (AAS) to analyze for and toxic compounds and X-Ray Fluorescence (XRF) for cations and toxic metals. The results of soil chemical analysis are tabulated and presented under results and discussion.

Water sample methods
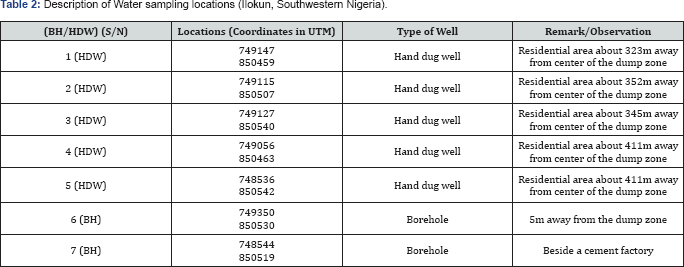
A total of seven (7) groundwater samples from two (2) boreholes (BH) and five (5) hand dug wells (HDW) were used for this study, with depth of 7.2m - 12.4m. Figure 3 shows the sampling points in the study area. The choice of the wells used for the study depends on the availability of wells in the study area. The samples was taken randomly from within the dump zone to the outskirt of the study area. The sampling was done in August 2017 and the Global Positioning Systems (GPS) readings of all the sampled boreholes and hand-dug wells were taken for accurate location (Table 2). The wells studied were of varying depths due to the topography of the site. Most of the boreholes were in a clean environment and had covers. Most of the hand- dug wells were also covered and had concrete ringlining, but a few were left open. The environmental conditions around the wells varied slightly (Table 2).
Soil geochemical analysis
The chemical parameters examined in the soil are, cations concentrations which includes potassium (K), calcium (Ca), Magnesium (Mg), Aluminum (Al) Titanium (Ti), Manganese (Mn), Iron (Fe), Arsenic (As), Zinc (Zn), Rubidium (Rb), Strontium (Sr), Zirconium (Zr), Niobium (Nb),Cerium (Ce),Phosphorus (P), Lead (Pb), Cadmium (Cd), Nickel (Ni), Copper(Cu), Chromium (Cr), Thorium (Th), Uranium (U), Barium (Ba) and Yttrium (Y) and theanions concentration which included those of Chloride ([Cl]A-), Sulphate ([SO]_4A(2-)), Nitrate ([NO]_3A-) and Phosphate ([PO]_4A(2-) ).
The cations concentrations were determined using X-Ray Fluorescence (XRF)(ECLIPSE ^)at the Centre for Energy Research and Development (CERD), Obafemi Awolowo University (OAU) Ile-Ife. The working procedure of the machine is such that a small sample will be irradiated are placed in the sample chamber. The sample chamber has connections to it, which are at angle [45]Aoto it respectively, the source X-ray tube and the Si-PIN photodiode detector. The X-ray source tube will eject beams of X-radiation onto the sample, there by irradiating the samples. The sample fluorescence's to give off characteristic X-ray of the particular absorbing atoms from which the X-ray photons are ejected. The ejected photons are from the Quantum Physical electronic transition between the K and L shells which gives a K(a) radiation and the one between K and M shell giving K(P) radiation. The difference in energy between the K-L shell and K-M shell electron transitions emit photons seemingly reflected in form of increase in the wave length of the detected X-rays as compared to the incident X-rays respectively. These detected photon energies are signatures corresponding to known elements with standard experimental energies to which the detected energies are compared for each atom in the sample. The emitted photons are picked up by the detector and their electronically corresponding signal currents are ported to the preamplifier. The multichannel analyzer converts the signal to data and then passes it on to the quantitative analysis software package on a desktop.
The concentration of Phosphate ([PO]_4A(2-) ) in the samples was determined with the use of VANADO-MOLYBDO- PHOSPHORIC ACIDCOLORIMETRIC METHOD. This method is based on the ability of ammonium molybdate indilute orthophosphate solution to react under acidic conditions to form a heteropoly acidmolybdo-phosphoric acid. In the method, yellow coloured vanado-molybdo-phosphoric acid was formed and the intensity of the yellow colour which is proportional to the phosphate concentration in the solution was then measured with a UV visible spectrophotometer [21].
Concentrations of sulphate ([SO]_4A(2-)) in the samples were determined using TURBIDIMETRIC METHOD. This method is based on the principle of formation of barium sulphate in the colloidal form by a sulphate in the presence of (acidified HCl) barium chloride. The process is enhanced in the presence of glycerol or other organic compound. The absorbance of the colloidal solution can be measured against a standard on UV visible spectrophotometer.
The concentrations of nitrate ([NO]_3A-) in the samples were determined by using the ULTRA VISIBLE SCREENING METHOD. 0.7218g of KNO salt dried at 105 C for 24hrs was 3 dissolved in distilled water and diluted to mark in a 1000mL standard flask. This was preserved with 2mL chloroform and then used to prepare calibration standards of 1mg/L to 40mg/L by dilution. 0.2mL of HCl was added to 10mL of each of the standard solutions and mixed thoroughly. Absorbances of the standards were then read against distilled water of zero absorbance at 220nm and 275nm. Differences in the readings were plotted against the concentrations to obtain calibration curve for the analysis. To 10mL of each sample, 0.2mL HCl was added and mixed thoroughly before reading the absorbance at 220nm and 275nm. The differences in absorbances were used in determining the concentration of nitrate in the samples from the calibration curve obtained.
Hydro-Chemical analysis
The chemical parameters examined in waters in the study area were, pH, total dissolved solids (TDS), electrical conductivity (EC), alkalinity, acidity, total hardness, cations concentrations which includes does of Iron (Fe), Magnesium (Mg), Zinc (Zn), Manganese (Mn), Calcium (Ca), Cadmium (Cd) Nickel (Ni), Chromium (Cr), Copper (Cu), Lead (Pb), Arsenic (As) and the anions concentration which included those of Chloride ([Cl]A. ), Sulphate ([SO]_4A(2-)),Nitrite ([NO]_2A-), Nitrate ([NO]_3A-) and Phosphate ([PO]_4A(2-) ).
The pH of the water samples was measured immediately after sample collection by using a checker pocket-sized pH meter with a replaceable electrode. The meter is a product of WOONSOCKET, RI 02895 HANNA. In order to obtain accurate results, the meter was first standardized with buffers of pH 7.00 and 4.00. The electrode of the meter was inserted into the water samples and the pH value was read directly on the meter. The total dissolved solids (TDS) of the water samples were determined with the use of M E T T L E R T O L E D O M C 1 2 6 TDS/conductivity meter. This instrument which is of average size with two sensitive probes was similarly used for measuring the electrical conductivity (EC) of the samples. To measure the TDS, the instrument was put on TDS mode while the probes were inserted into each sample and the displayed TDS value was read. Conversely, to measure the electrical conductivity of the samples the meter was changed to EC mode and first standardized with a solution of electrical conductivity of 12.88us/cm at 25 C in order to obtain accurate results. For each analysis of EC, the probes of the meter were inserted into the water sample such that the water level was above the probes. The instrument measures TDS ineither milligram per litre (mg/L) or gram per litre (g/L) while the EC is measured in siemens (S) or microsiemens (uS). Akalinty was determined in the water sample by using Titrimetric method, Total Acidity was determined by using AOAC method while the concentration of Total Hardness is determined by Titration method. The cations concentrations were determined using Atomic Absorption Spectrophotometer (AAS) (Buck scientific 210 VGP) at the Centre of Research and Extension, Afe Babalola University, Ado- Ekiti (Abuad). The anions concentration which included those of Sulphate ([SO]_4A(2-)),Nitrite ([NO]_2A-), Nitrate ([NO]_3A-) and Phosphate ([PO]_4A(2-) ) were determined at sustainable laboratory service, Akure. The concentration of Chloride ([Cl]A. ) were determined by Argentometric method, concentration of phosphate ([PO]_4A(2-)) samples was determined with the use of Vanadomolybdo-phosphoric acid colorimetric method, concentrations of sulphate ([SO]_4A(2-)) in the water samples were determined using Tutbidimetric method and while the concentrations of nitratein the water samples were determined by using the Ultra-visible screening method.
Results and Discussion
Soil geochemical results
The results of the geochemical analysis of the soil in and around Ilokun waste dumpsite environment were shown in Table 3a & 3b) and were represented by plots shown in Figure 4.
The concentration level of cations and anions such as Ca, Ti, Mn, Fe, Nb, Ce, Pb, Cd, Cu, Ba, Cr,U, Mg,[Cl]A-,[NO]_3A-, [PO]_4A(3-)and [SO]_4A(2-) from S1 to S12 where generally lower than the control point S13. The higher concentration in S13 of some anions and cations may be due to the anthropogenic activities and the geology of the area.

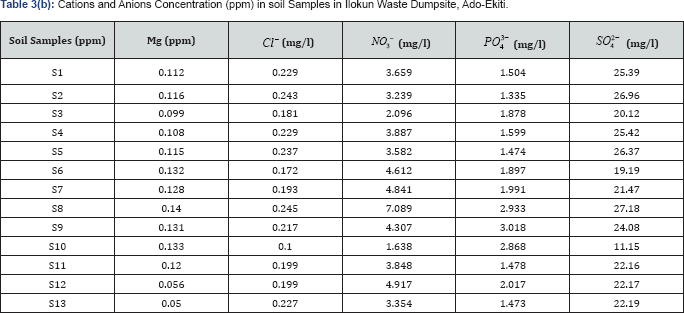

While cations concentrations of K, As, Zn, Rb, Sr, Zr, P, Ni, Th and Y from S1 to S12 are highergenerally as compared to the control point S13. Which indicate contamination of the topsoil and the madeup soil by the waste dumpsite.
The concentration values of K ranges from 542 to 1065ppm, As ranges from 70 to 1131ppm, Zn ranges from 166 to 1257ppm, Rb ranges from 113 to 1094ppm, Sr ranges from 251 to 714ppm, Zr ranges from 1500 to 12914ppm, P ranges from 24 to 161352ppm, Ni ranges from 500 to 2444ppm, and Th ranges from 441 to 1034ppm as against the control point concentration of 488ppm, 25ppm, 125ppm, 63ppm, 100ppm, 1471ppm, 0ppm, 424ppm and 421ppm.
Also the concentration value of Y ranges from 42ppm to 219ppm and no concentration of Ywas not detected at the control point.
The concentration values of K ranges from 542 to 1065ppm. The soil in and around the waste dumpsite is rich in potassium due to the impact of the waste dump and possibly by the application of N-P-K fertilizers by farmer on the surrounding soil which enhance the potassium content in soil direct contact of soils that are contaminated with lead may pose a human health risk according to Amadi and Nwankweala [22].
Arsenic is a toxic element widely encountered in the environment and in organisms. The concentration in the study area ranges from 70 to 1131ppm. Arsenic can enter terrestrial and aquatic environments through both natural formation and anthropogenic activities [23]. Persistence of arsenic within soil and its toxicity to plants and animals is of concern. Long-term exposure to high concentrations of As can lead tochronic arsenic poisoning (arsenicosis). Gastrointestinal tract, skin, heart, liver and neurological damage. Diabetes, Bone marrow, blood diseases and Cardiovascular disease. Expose to exposure arsenic is mainly through consumption of groundwater containing naturally high levels of inorganic arsenic, food prepared with this water, or food crops irrigated with water high in arsenic.
Zinc toxic effects in humans are most obvious from accidental or occupational inhalation exposure to high concentrations of zinc compounds, such as from smoke bombs, or metal- fume fever [24]. The concentration for Zn ranges from 166 to 1257ppm. Modern occupational health and safety measures can significantly reduce potential exposure. Intentional or accidental ingestion of large amounts of zinc leads to gastrointestinal effects, such as abdominal pain, vomiting and diarrhoea. In the case of long-term intakes of large amounts of zinc at pharmacological doses (150-2000mg/day), the effects (sideroblastic anaemia, leukopenia and hypochromic microcytic anaemia) are reversible upon discontinuation of zinc therapy and/or repletion of copper status, and are largely attributed to zinc-induced copper deficiency [25].
Rubidium could be gotten from glasses ceramics and engines for space vehicles waste. The concentration of Rb ranges from 113 to 1094ppm Rubidium has no known biological role but has a slight slimulatory effect on metabolis, probably because it is like potassium. The two elements are found together in minerals and soils, although potassium is much more abundant than rubidium. Plant will adsorb rubidium quite quickly. When stresses by deficiency of potassium some plants, such as sugar beet, will respond to the addition of rubidium. In this way rubidium enters the food chain and so contributes to a daily intake of between 1 and 5 mg. If rubidium ignites, it will cause thermal burns. Rubidium readily reacts with skin moisture to form rubidium hydroxide, which causes chemical burns of eyes and skin. Signs and symptoms of overexposure: skin and eye burns. Failure to gain weight, ataxia, hyper irritation, skin ulcers, and extreme nervousness. Medical condition aggravated by exposure: heart patients, potassium imbalance [25].
Strontium is tow kind stable and radioactive element. When you eat food or drink water containing strontium. The concentration of Sr ranges from 251 to 714ppm, Strontium acts very much like calcium [26]. Strontium mostly attaches to the surfaces of bones and bones marrow bone itself and nearby soft tissues may be damaged by radiation released over time and caused anemia. Cancers of the bone and a decreased resistance to fight disease. Damage to the genetic materials in cells [27].
Although the toxic effects of Zr on plants, especially on root growth, are commonly reported, its stimulating effect on the growth of yeasts and on metabolism of other microorganisms. Zr to be the least toxic element, among the heavy metals, to barley seedlings. He described that Zr treatment enhances protein synthesis andchanges the amino acid composition of the proteins of some microfungi, but also reduces the phytoavailability of phosphates to phytoplanktons.
The concentration of P ranges from 24 to 161352ppmIncreases in the generation of organic wastes, in addition to decreases in natural resources, make it necessary to recycle these waste materials. In soils, the application of wastes as organic fertilizers, allows to reincorporate nutrients to the biogeochemical cycles. The application of wastes in agriculture has carried out with it problems associated with phosphorus (P) over application in soils and contamination of water bodies. Phosphorus inside the waste matrix forms organic and inorganic compounds which have different bio availabilities. The more important factors that influence the bioavailability of P in soils, organic wastes and soil- organic waste amendments are: the soil solution pH, adsorption reactions, organic matter, phosphatase activity and low molecular weight of organic acids. In the wide range of organic wastes, purines and compost have been selected to illustrate the effects of the factors previously mentioned on the bioavailability of P in soils [28].
Phosphorus in its pure form has a white colour. White phosphorus is the most dangerous form of phosphorus that is known to us. When white phosphorus occurs in nature this can be a serious danger to our health. White phosphorus is extremely poisonous and in many cases exposure to it will be fatal. In most cases people that died of white phosphorus exposure had been accidentally swallowing rat poison. Before people die from white phosphorus exposure they often experience nausea, stomach cramps and drowsiness. White phosphorus can cause skin burns. While burning, white phosphorus may cause damage to the liver, the heart or the kidneys.
The concentration of Ni in the study area ranges from 500 to 2444ppmNickel is present in a number of enzymes in plants and microorganisms. In humans, essential component of the haemopoietic process, and play important role in physiological processes as a co-factor in the absorption of iron from the intestine. Involved the human immune system. The reference values for nickel in healthy adults are 0.2|ig/L in serum and 1-3|ig/L in urine [29]. Zinc deficiency caused of human body will suffer from hair and memory loss, skin problems, weakness in body muscle problems during pregnancy also causes stunted brain development of the fetus and exists of nickel caused of decreased body weights, significantly increased heart and decreased liver weights the carcinogenicity of nickel compounds which occurs through inhalation mainly as a result of occupational exposures.
Thorium is a naturally occurring radioactive element that is widely distributed in the crust of the Earth. This element is very common in mineral formations in regions with high levels of radioactivity Thorium is ubiquitous in our environment. The concentration of Th ranges from 441 to 1034ppm. Release of thorium can occur both from natural and anthropogenic sources. Data on the fate and transport of thorium in the air are limited but wet and dry deposition seems to be involved in removal from atmosphere. Surface water sediment is the repository for atmospheric and aquatic compounds. In water, the concentration of soluble thorium is low (1.10-5g/l in sea water), thorium is present in sediment and suspended particles. It has been shown that significant bio concentration occurs in lower trophic animals in water, but bio concentration factors decrease as trophic level increase. In soil, thorium remains strongly sorbed in most cases and its mobility will be slow. However, in some soils, thorium can form soluble complexes and leach into groundwater. The plant/soil transfer ratio for thorium is <0.01, thorium does not concentrate in plants from soil. But, in contaminated areas, this ratio can reach 3 [30]. Industrial waste is the main source of thorium to the environment caused by ionizing radiation due to thorium are those involved in mining and processing of rare-earth and phosphate containing ores, in pyrochlore mining and niobium processing, and in the manufacturing of gas mantles, high-intensity discharge lamps and thorium oxide manufacturing welding rods.
The transfer of radionuclide's from the soil to organic materials determines the extent of radioactive contamination of food and plants and thus the risk of radiation exposure of the population due to food intake. Radiological studies have shown that some eco systems have conditions that favor transfer of radionuclide's from the soil to organic material [31].
Yttrium is dumped in the environment in many different places, mainly by petrol producing industries. It can also enter the environment when household equipment is thrown away. Yttrium will gradually accumulate in soils and water soils and this will eventually lead to increasing concentrations in humans, animals and soil particles [32].
The concentration value of Y ranges from 42ppm to 219ppm. Yttrium is one of the rare chemicals, that can be found in waste generated from home used equipment such as colour televisions, fluorescent lamps, energy-saving lamps and glasses. All rare chemicals have comparable properties. Yttrium can rarely be found in nature, as it occurs in very small amounts. Yttrium is usually found only in two different kinds of ores. The use of yttrium is still growing, due to the fact that it is suited to produce catalysers and to polish glass. Yttrium is mostly dangerous in the residential environment, due to the fact that damps and gasses can be inhaled with air. This can cause lung embolisms, especially during long-term exposure. Yttrium can also cause cancer with humans, as it enlarges the chances of lung cancer when it is inhaled. Finally, it can be a threat to the liver when it accumulates in the human body.
Hydro-chemical results
The temperature, pH, total dissolved solids (TDS), electrical conductivity (EC), total acidity, alkalinity and total hardness of the ground water (hand-dug wells and boreholes) sampled are shown in Table 4. On the basis the general classification of water recommended by NSDWQ and WHO International Standard for drinking water in 2007 and 2011 respectively (Table 5). As shown in Table 4, the concentration values of electrical conductivity (EC) and total hardness in all the water samples are within the NSDWQ and WHO standard for drinking water Temperature values in the samples ranges from 26.5(_Ao)C to 29.5(_Ao)C are higher than the WHO standard. All the water samples tested, their pH and TDS were within the recommended range of between 6.5 to 9.5 and 500[mgl]A(-i)respectively which is the allowable concentrations for drinking water, except for the concentrations of 2(HDW) and 3(HDW) which is not within the recommended range and are slightly acidic. The total acidity concentration of water in the study area increases near the waste dumpsite and decreases away from the dumpsite. The alkalinity concentration in water in the area ranges 120.0 to 232.0 mgCaCO3/l which exceeds the maximum required standard for drinking water. The elevated TDS total acidity and alkalinity values in the water in the study area are an indication of the presence of inorganic salts (principally Ca, Mg, K, bicarbonates chlorides and sulfates) [33].


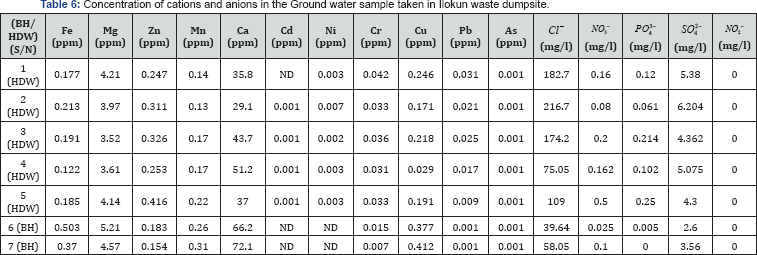
The results of analyzed major cations and anions as reported in Table 6 reveals that the concentration levels of all the cations and anions that were analyzed in the water samples in the study area were relatively low and are within the NSDWQ and WHO maximum permissible limits. Except for Fe which has a concentration value of 0.503ppm in 6(BH) which higher than the recommended values. 6(BH) is 2m away from the dumpsite as indicated in Table 2. The high concentration of iron in this samples can be attributed to the closeness of the waste dumpsite.
The magnesium (Mg) concentrations of the ground water samples ranged from 3.520 to 5.212ppm. These values exceeds maximum permissible levels (0.20ppm) for potable ground water recommended by NSDWQ [1]. These high values of magnesium in the water samples may indicate that water in these wells have has been infiltrated by toxic materials from the waste dumpsite.
Manganese (Mn) concentration in the water samples ranges from 0.128 to 0.313ppm. This range of values exceeds the maximum permissible levels of 0.01 to 0.05ppm. The high presence of manganese may be due to discharge from industrial facilities or as leachate from landfills The very high values of manganese may be as a result of pollution from manganese dioxide cells for which the nation has no controlled methods of disposal. The metal may also come from other sources such as domestic wastewater and sewage sludge disposal [16].
The concentrations of lead (Pb ) in 1(HDW), 2(HDW), 3(HDW) and 4(HDW) are 0.031ppm, 0.021ppm, 0.025ppm and 0.017ppm respectively which exceeds of maximum permissible levels of 0.01ppm. This is due to the closeness of the waste dumpsite and may be possibly located in the direction of the leachate plume. While 5(HDW), 6(HDW) and 7(HDW) are below the maximum permissible levels for quality drinking water. Lead in the environment is mainly particulate bound with relatively low mobility and bioavailability. Lead does, in general, not bio accumulates and there is no increase in concentration of the metal in food chains. Lead is also not essential for plant and animal life.
The presence of lead in the water may be due to the discharge of industrial effluents from petroleum production. Lead may also come from lead-acid batteries, plastics and rubber remnants, lead foils such as bottle closures, used motor oils and discarded electronic gadgets including televisions, electronic calculators and stereos],where leachates from the waste dumpsites may find their way into groundwater in the study area.
Conclusion and Recommendation
The soil in Ilokun and its environs, South western Nigeria is relatively free of hazardous cations and anions such as Pb, Cd, Cr, U, Mg, [Cl]A-,[NO]_3A-,[PO]_4A(3-)and [SO]_4A(2-) as indicated in all the samples analyzed. While hazardous cations such as As, Ba, Thr and Zn has relatively high concentration in and around the area of the dumpsite. This indicate that the topsoil and the made up soil in the area are completely contaminated.
The groundwater in the region of 2(HDW) and 3(HDW) are slightly acidic as indicated by the relatively high pH and EC concentration values. The high acidic concentration in this hang- dug wells is due to fact that they are located on or beside the direction of leachate plume flow which originates from the waste dumpsite. The levels of some cations and anions such as Zn, Ca, Cd, Ni, Cr,As,[Cl]A- [NO]_3A- [PO]_4a(3-), and [SO]_4A(2-) in the studied groundwater samples confirmed to the NSQDW and WHO recommended for drinking water.
However the concentration of cations such as Fe, Mg, Mn and Pb generally exceed the NSQDW and WHO standards. Which is attributed to leachate flow from the dumpsite to the groundwater in Ilokun environs.
Hence, it can be concluded that most of the groundwater samples from ilokun area, are not potable and good for human consumption on the basis of the high concentrations of toxic metals that exceed WHO standards in most of the samples studied.
However, since the majority of people living in this area depend on the soil for farming and water supply from the hand- dug wells and boreholes for domestic purposes, there is the need to alert the local authority in the area of this dangerous trend, so that an alternative arrangement can be made to provide potable domestic water for the residents of the area. We also recommend that waste dumpsite should be discontinued and located at the outskirt of the town by the local authority and thorough study of the topography of the location of new boreholes and hand dug wells should be considered to avoid contamination of soil and ground water from sources such as surface erosion of fertilizers and septic tanks.
References
- Nigerian standard for drinking water quality. Nigeria Industrial Standard, Approve by Standard Organization of Nigeria Governing Council. ICS 13. 060. 20: 15-19.
- World Health Organization (WHO) (2011) Guidelines for drinking- water quality recommendation, Geneva, p 1-6.
- Lateef TA, Eluwole AB, Adewa DJ (2015) Geoelectrical assessment of the impact of the Ilokun dumpsite, Ado-Ekiti Southwestern Nigeria, on surrounding groundwater Aquifers. International Letters of Natural Sciences 40: 41-47.
- Pushpentra SB, Anjana S, Akhilesh KP, Priyanka P, Abhishek KA (2012) Physico-chemical analysis of groundwater near municipal solid waste dumping sites in Jabalpur. International Journal of Plant, Animal and Environmental Sciences 2(1): 217-222.
- Papadopoulou M, Karatzas G, Bougloukou G (2006) Numerical modeling of the environment impart of landfill lonhage on groundwater quality a field application. Greece Journal of Science 5(3): 67-78.
- Hoornweg D (1999) Solid waste management in Asia. The international Bank for Recombination and development, World Bank report, pp. 100-167.
- Adeniran AA, Adewole AA, Olofa SA (2014) Impact of solid waste management on Ado -Ekiti property values. Journal of Civil and Environmental Research 6(9): 29-38.
- Akinola P (2016) Investigation of the implementation and effectiveness of electronic waste management in Nigeria. Model Earth SystEnviron 2(100): 1-6.
- Visvananltan L, Gcawe U (2006) Domestic solid waste management in south Asian countries. A comparative analysis. Journal of Environmental Science 3(2): 245-264.
- Abdus-Salam N, Ibrahim MS, Fatoyinbo FT (2011) Dumpsites in Lokoja, Nigeria. A silent pollution zone for underground water. Journal of Waste management and Bio-resources technology 3(1): 21-30.
- Bayode S, Adeniyi KE (2014) Integrated geophysical and hydrochemical investigation of pollution associated with the Ilara-mokin dumpsite, South-western Nigeria American international. J Contemp Res 4(2):150-161.
- Adebayo AS, Ariyibi EA, Awoyemi MO, Onyedim GC (2015) Delineation of contamination plumes at Olubonku dumpsite using geophysical and geochemical approach at Ede Town, Southwestern Nigeria. Geosciences 5(1): 39-45.
- Raman N, Warayanan (2008) Impact of solid waste effort in groundwater and soil quality near to pallavarem solid waste landfill site in Chennai. Indian Journal of .Chemical Sciences 1(4): 828- 836.
- Byoung-Young C, Seong-Tack Y, Soon-Young Y, Pyoung-koo L, Seoung- Sook P, et al. (2005) Hydrochemistry of urban groundwater in Seoul, South Korea: Effect of land -use and pollutant recharge. Environmental Geology 48: 979-990.
- Kassenga GR, Mbuligwe SE (2009) Impart of solid waste disposal site on soil, surface water and Ground water quality in Dar-es salaam City, Tanzania. Journal of Sustainable Development in Africa 10(4): 73-94.
- Nartey VK, Hayford EK, Ametsi SK (2012) Assessment of the impact of solid waste dumpsite on some surface water system in the Accra Metropolitan Area Ghana. Journal of water Resource and Protection 4(8): 605-615.
- George NJ, Ubom AI, Ibanga JI (2014) Integrated Approach to Investigate the Effect of Leachate on Groundwater around the Ikot Ekpene Dumpsite in Akwa Ibom State, Southeastern Nigeria. International Journal of Geophysics 2014: 12.
- Olagunju OE, Ariyibi AE, Awoyemi MO, Adebayo AA, Dasho OA, et al. (2017) Application of geochemical and geophysical approach to environmental impact assessment: a case study of Emirin active open dumpsite. Ado-Ekiti Southwestern NigeriaModel Earth Syst Environ p: 1-13.
- Akinyemi SA (2013) Mineralogy, physicochemical characteristics and industrial potential of some resident clay deposits within Ekiti-state, southwestern Nigeria. Journal of Environment and Earth Science 4(17): 70-88.
- Rahaman MA (1976) Review of the basement geology of Southwestern Nigeria. In: Geology of Nigeria, Kogbe CA (Eds.), Elizabethan Publishing Co: Lagos, Nigeria, p. 41-58.
- Olorunfemi AO, Salahudeen KS, Adesiyan TA (2011) Groundwater quality in Ejigbo town and environs, Southwesthern Nigeria. IfeJ Sci 13(1):111-119.
- Amadi AN, Nwankweala HO (2013) Evaluation of heavy metal in soils from enyimba dumpsite in Aba southeastern Nigeria, using contamination factor and geo-accumulation index. Energy and Environment Research 3(1): 130-142.
- Tu C, Ma LQ (2003) Effects of arsenate and phosphate on their accumulation by an arsenic-hyperaccumulator Pteris vittata L. Plant and Soil 249(2): 373-382.
- El-Sayed EO (2016) Environmental modelling of heavy metals usingpollution indices and multivariate techniques in the soils of Bahr El Baqar, Egypt. Model Earth Syst Environ 2(119): 1-17.
- Lars Järup (2014) Hazad of heavy metal contamination. Department of Epidemiology and Public Health, Imperial College, London, UK.
- Martine CS (2002) Strontium Overload and toxicity: impact on renal osteodystrophy, Nephronl Dial Transplant 17: 30-34.
- Lenntech BV (2015) Health Effects of elements.
- Bárbara F, Nanthi B, Ravi N, María M (2006) Phosphorus in organic waste-soil systems. J Soil Sc Plant Nutr 6(2): 64-83.
- Hafeezl B, Khanif YM, Saleem M (2012) Role of Zinc in Plant Nutrition a Review, American Journal of Experimental Agriculture 3(2): 374-391.
- Le Guen B, Bérard P, P L (2000) Thorium. Encycl Méd Chir (Editions Scientifiques et Médicales Elsevier SAS, Paris, to us droits réservés), Toxicologie-Pathologie professionnelle, 16-010-A-10, p. 9.
- Mychelle MR, Vera AM, Maria HT, Marco AS, Marcelo TF (2011) Determination of 228Th, 232Th, and 228 Ra in wild mushroom from a naturally high radioactive region in Brazil. 2011 International Nuclear Atlantic Conference - INAC 2011, Belo Horizonte, MG, Brazil, ASSOCIAÇÂO BRASILEIRA DE ENERGIA NUCLEAR-ABEN.
- Ajugwo AO (2013) Negative effects of gas flaring: The Nigerian experience. Journal of Environment Pollution and Human Health 1(1): 6-8.
- Ayolabi EA, Folorunso AF, Kayode OT (2013) Integrated geophysical and geochemical methods for environmental assessment of municipal dumpsite system. International Journal of Geosciences 4: 850-862.






























