The Efficiency Evaluation of Applying Poly Ferric Sulfate and Perlite for Filtration
Ali Reza Taheri Fard*
Department of Civil Engineering, Saint Petersburg Polytechnic University, Russia
Submission: September 16, 2017; Published: December 07, 2017
*Corresponding author: Ali Reza Taheri Fard, Department of Civil Engineering, Peter the Great St. Petersburg Polytechnic University, 29 Politechnicheskaya St., St. Petersburg, 195251, Russia, Email: alireza_taherifard@yahoo.com
How to cite this article: Ali R T F.The Efficiency Evaluation of Applying Poly Ferric Sulfate and Perlite for Filtration. Civil Eng Res J. 2017; 2(5): 555599. DOI: 10.19080/CERJ.2017.02.555599
Abstract
The horrible quality of water from sand filters leads us to study on the basis and sieving for this sort of filtration. In addition, we assessed surface charges in the style of 4 samples regarding chemical treatment from two areas before going to water treatment plant and from output. Our inspection demonstrated that the combination of perlite and certain dosage of poly ferric sulfate with the filter surface 60% of surface charge shows desirable efficiency.
Keywords: Ferric; Perlite; Turbidity; Liquor; Urbanaization
Abbrevations: Al: Aluminum; Fe: Iron; PACI: Poly Aluminum Chloride; DOC: Dissolved Organic Carbon
Introduction
Growing population, improving of living standards, urbanization, industrial development and agriculture are the main factors that increased water consumption and waste water production in the community, consequently giving rise to environmental pollution [1,2].
Filtration is a separation process that consists in passing a solid-liquid mixture through a porous material (filter) which retains the solids and allows the liquid to pass through [3,4]. Removing suspended solids by high-rate granular filtration is a complex process involving a number of phenomena. Attempts to develop theories that quantitatively predict solids removal performance with sufficient precision and versatility to be of use in practical filter design have met with relatively little success. Consequently, filter media selection is often an empirical process. Pilot investigations are common tools for assessing the performance of a particular filter design [5-7].
The used process for water treatment depends on the quality of water resource. Surface water normally has more variable pollutants compared with underground water, despite the fact this surface water could do with more complicated treatment process [8,9]. The most surface water in the world has more pollutants and turbidity compared with standards for potable water. Although, high velocity of water stream might have more suspended solids, the most of them are in colloidal size. Hence, coagulation and filtration are good choice for treatment [10-12].
The first extensive use of perlite was for filtration of raw cane sugar liquor as early as 1876 [13]. Today the primary industrial application of diatomite is as an industrial filtration medium for liquids ranging from municipal water supplies to alcoholic beverages. In contrast, substantial commercial production of perlite did not begin until 1846. In 1963 only 15 percent of the perlite produced in the United States was used as filter media [14].
Light weight expanded perlite structures are milled and classified using strictly defined processes to produce perlite filter aids with specific flow characteristics. The various grades utilize the jagged inter locking structure to create billions of micro-scopic channels between the filter aid particles to produce optimum flow rates and clarification ability for a wide variety of applications [15].
Perlite filter aids are light weight, inert, impart no taste or odor to liquids being filtered and are virtually in soluble in mineral and organic acids at all temperatures, solubility in strong alkaline solutions vary depending on temperature and contact time [16]. Without using the filter aids the solid particles in liquid will soon accumulate on filtering surfaces and block them. A perlite filter aid makes a filtering layer that transfers the actual filtering from the septum to the whole mass of filter aid. Filtration occurs in the tiny pores formed by the fine particles of filter aid [17].
This study aims to find effective factors on efficiency of filtration in order to enhance efficiency of physical and chemical parameter and most importantly reducing turbidity [18].
This study presents the recent laboratory research related to the application of engineered nano particles consisting of in organic phases in water treatment and their classification in the fields of heavy metal removal, anti microbial activity and organic compound degradation. The reported results are discussed not only according to their potential for application in different drinking water treatment processes but also from a critical consideration of the possibility to scale-up in technologically viable methods and become competitive with existing techniques and conventional materials. As one of the main limitations in the effort to evaluate the efficiency of nano materials from different authors is the absence of a unified procedure that enables direct comparison of results, this work suggests an experimental methodology working with reliable conditions and parameter ranges of drinking water treatment and generating proper indices for the validation of performance.
Aluminum (Al) and iron (Fe) salts are two widely used coagulants. Large flocs are formed in water sub sequent to hydrolysis of the metal-based coagulants and then settled in a sedimentation tank. The floc properties (e.g., size, density and strength) determined by coagulant species, chemical dosage, and solution chemistry significantly influence coagulation efficiency. Further, the coagulation performance can greatly affect the membrane process For example, small floc sizes lead to high cake resistance on membranes. Prior efforts were made to determine an optimal coagulant dosage to minimize membrane fouling. Lee et al. found an optimal poly aluminum chloride (PACl) dosage with respect to fouling minimization, and the dosage depended heavily on the physical and chemical characteristics of the waste water. Tran et al. reported that dissolved organic carbon (DOC) in water could be significantly reduced with a specified dosage of Al to reduce membrane fouling.
Methods
Due to the fact that changing materials on the basis of filtration is much expensive, we assumed the part of it as our model. The evaluated parameters were turbidity, TSS, total solids, the amount of Ca and Mg. we got our samples from two area first was the water which goes to water treatment plant and second was in output of water treatment plant.
The methods considering the experiments divided into different stages which each of them implies divergent episodes. First step was done without any coagulation, we carried out our experiments on filtration soon after sedimentation and we attained the chemical properties after our experiments on two samples and we call this step A. Second step was done with using poly ferric sulfate as our best coagulants, we used poly ferric sulfate with dosage of 8ppm as the optimum dose, several hours later of jar test, we implemented filtration experiment on this sample and attained the results on chemical properties and we named this step B. Next step was done with combination of using perlite and anthracite, in this step we added perlite with anthracite on the surface of filtration, we arranged the array of them respectively from upside to down part, perlite 48cm (ranging of sieving 0.5-2mm), anthracite 45cm (1-3mm), perlite 15cm (4-6mm), perlite 24cm (5-8). After making adjustment on grading we performed our experiments and attained the results and we named it C. The last step was done similar to prior one but with the difference of height of used materials in filtration, we changed the surface charge and reduced it up to 60% of our last experiments, after making some alteration on grading and surface charges we attained our results and we named it D.
Results
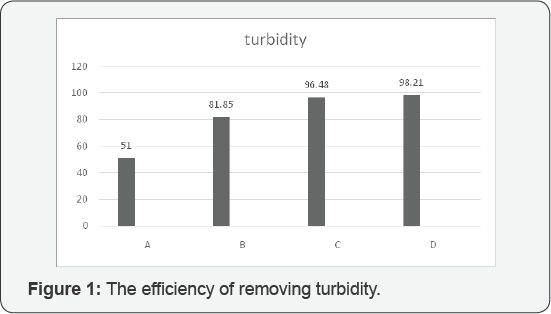
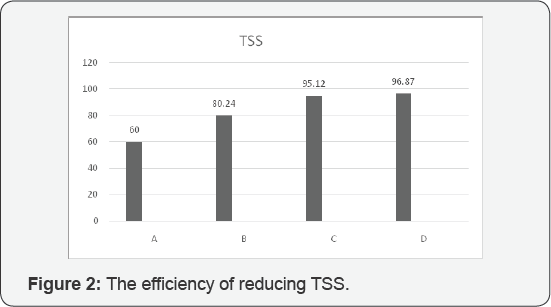
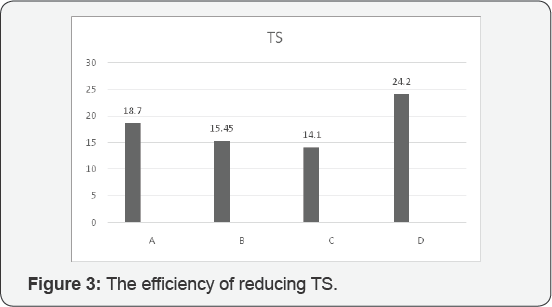
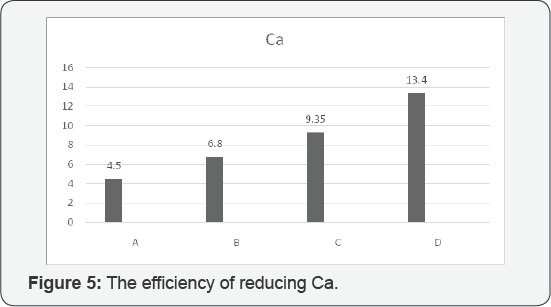
According to our evaluation and analysis of experiments, these are being claimed that the best efficiency of turbidity assigned to C and D respectively 96.48% and 98.21%. The best efficiency for reducing TSS was assigned to D with 96.87 %, moreover, the best efficiency for reducing total solids was allocated to D with 24.2%, the best efficiency for reducing Ca was assigned to D with 13.4 %. And also fourth stage succeeded to get the best efficiency of reducing Mg with 26.5 %. (Figure 1-5).

Conclusion
Pursuant to our results in analyzing our samples considering the parameters TSS, turbidity, total solids, Mg and Ca, it clearly shows that this method is pretty impeccable if we use it properly in the case of applying poly ferric sulfate for coagulation, it gives rise to boost the quality of water as it is depicted in charts as well as reducing turbidity as well as decreasing the amount of fees for removing disinfection with considering PH and temperature and another metals materials in the water. In compared with current methods in scientific world, this method and results demonstrate the new idea and method in order to get standard quality according to US EPA.
References
- Eddi Yu Tszen (2015) Novaya stranitsa po zagryazneniyu okruzhayushchey sredy. Zagryazneniye okruzhayushchey sredy 204: A1.
- Ceyhun E, Oguz O (2014) Zagryazneniye i neformalnaya ekonomika. Ekonomicheskiye sistemy 38(3): 333-349.
- Zouboulis AI, Traskas G (2005) Sravnitelnaya otsenka razlichnykh kommercheski dostupnykh koagulyantov na osnove alyuminiya dlya obrabotki poverkhnostnykh vod i dlya posleduyushchey obrabotki gorodskikh stochnykh vod. Zhurnal khimicheskoy tekhnologii i biotekhnologii 80: 1136-1147.
- Degremont O (2002) Spravochnik po vodopodgotovke 6-ye izd. Lavuazye Uayli Nyu-Dzhersi.
- AWWA (2004) Kachestvo vody i lecheniye. 4-ye izd. Amerikanskaya assotsiatsiya vodoprovodnykh rabot MakGrou-Khill Nyu-York.
- Barut EE (2005) Proyektirovaniye vodoochistnykh sooruzheniy 4-ye izd. MakGrou-Khill Nyu-York.
- Montgomeri JM (1985) Printsipy i dizayn vodoochistki. John Wiley & Sons Nyu-Dzhersi.
- Tarique A, Kafeel A, Abdul A, Mehtab A (2016) Kharakteristika osadka dlya obrabotki vody i yego povtornoye ispolzovaniye v kachestve koagulyanta. Zhurnal ekologicheskogo menedzhmenta 182: 606-611.
- Molodkina LM, Kolloidnaya KV (2010) usloviyakh bezopasnosti vodnykh sistem Kolloidnaya khimiya v oblasti bezopasnosti vodnykh siste. 205.
- Devi R, Alemayehu E, Singh V, Kumar A, Mengistie E (2008) Udaleniye ftoridnykh myshyakovykh i koliformnykh bakteriy s pomoshchyu modifitsirovannykh samodelnykh filtruyushchikh sred iz pityevoy vody. Bioresursnyy tekhnol 99 (7): 2269-74.
- Ho L, Grasset C, Hoefel D, Dixon MB, Leusch FDL, et al. (2011) Otsenka filtratsii granulirovannykh sred dlya udaleniya khimicheskikh zagryazniteley iz stochnykh vod Water 45 (11): 3461-72.
- Remize PJ, Laroche JF, Leparc J, Schrotter JC (2009) Sravneniye pilotnykh granulometricheskikh filtrov i membrannoy filtratsii nizkogo davleniya dlya predvaritelnoy obrabotki morskoy vody. Opresneniye vody 5: 6-11.
- Petkof, Bendzhamin D (1965) Byulleten ministerstva vnutrennikh del Soyedinennykh Shtatov 630: 313-319.
- May, Timoti KP (1965) Byulleten ministerstva vnutrennikh del Soyedinennykh Shtatov 630: 655-661.
- Maksim, L. Daniel, Nibo, Ron; Makkonnell et al. (2014) Perlitovaya toksikologiya i epidemiologiya obzor. Ingalyatsionnaya toksikologiya 26(5): 259-270.
- Kim A., Chernikov N (2015) Uluchsheniye kachestva vody putem dopolnitelnoy filtratsii cherez sorbtsionnuyu nagruzku modifitsirovannuyu fullerenami. Prikladnaya mekhanika i materialy 725-726: 1338-1334.
- Perlit (2006) Geologicheskaya razvedka SShA. Mineralnaya syryevaya svodka pp. 122-123.
- SS UluatamOtsenka (1991) perlita kak zamenitelya peska v filtratsii AWWA p. 83.






























