The Inexplicable Drop in Intraocular Pressure through the Trabecular Meshwork Explained. The Unsuspected Ability of the Eukaryotic Cell to Dissociate the Water Molecule
Arturo Solís Herrera*, María del Carmen Arias Esparza and Paola Eugenia Solís Arias
Human Photosynthesis™ Study Center, Aguascalientes, México
Submission: July 31, 2023; Published: August 15, 2023
*Corresponding author: Arturo Solís Herrera, Human Photosynthesis™ Study Center, Aguascalientes, México Email: comagua2000@yahoo.com
How to cite this article: Arturo Solís H, María del Carmen Arias E, Paola Eugenia Solís A. The Inexplicable Drop in Intraocular Pressure through the Trabecular Meshwork Explained. The Unsuspected Ability of the Eukaryotic Cell to Dissociate the Water Molecule. Anatomy Physiol Biochem Int J: 2023; 6(5): 555699. DOI: 10.19080/APBIJ.2023.06.555699.
Abstract
Loss of vision caused by glaucoma is one of the three main causes worldwide. That is why researchers, clinicians, and patients make strenuous efforts to find an explanation for a disease that seems to resist both medical and surgical treatments. Glaucoma is a disease that was described from the Greeks, they called it the green disease. And despite the thousands of years that have passed, glaucoma is still a scourge of modern society, which means that available treatments aren’t working adequately.
The dynamics of the aqueous humor, considered an important basis of the biology of the eye, is shrouded in mysteries. In this work we will refer to one, and that could not be explained to date. Aqueous humor flows through the outflow network at an extremely low flow rate (2.0 μL/min), yet this flow produces a surprisingly large pressure drop over a short flow distance (less than 1 mm). The pressure drop is around 6 mmHg in normal eyes and can be as much as 40 mmHg in glaucomatous eyes.
That such a small flow and in a minimum distance and that it results in a marked drop in intraocular pressure is inexplicable. The various theories and mathematical models that have been proposed for its explanation have not succeeded. Our discovery of the unsuspected capacity of the eukaryotic cell to dissociate the water molecule opens a completely new horizon in this regard.
Keywords: Anterior Chamber; Angle; Iris; Schlemm’S Canal; Trabecular Meshwork; Oxygen; Water
Introduction
In the anatomy of the eye, we have an area less than one mm thick, in the shape of an isosceles triangle, which forms a circle that surrounds the cornea, located between the sclera and the cornea (Figure 1). It is called the trabecular meshwork and one of its components is called Schlemm’s canal. The diagram in Figure 1 is to demonstrate the location of a triangular-shaped anatomical structure, whose vertex would be formed by Schwalbe’s line, which is where the corneal endothelium ends, and whose base would be the scleral spur.
Said structure handles 90% of the aqueous humor that is produced in the ciliary body, and what is striking is its minimum size and flow, and yet its effect on intraocular pressure is decisive, since it is what maintains the intraocular pressure. intraocular within normal limits, that is: between 12- and 20-mm Hg. The explanation of how this IOP modulation occurs is something that is not understood, at least until before our discovery about the unsuspected capacity of the eukaryotic cell to dissociate the water molecule through various molecules, such as plants. In mammals, the most efficient molecule is melanin.
The Trabecular meshwork and Schlemm´s channel (Latin: sinus venosus sclerae)
It is an enigma of several decades, that aqueous humor flows through the outflow network at an extremely low flow rate (2.0 μL/min), yet this flow produces a surprisingly large pressure drop over a short flow distance (less than 1 mm). The pressure drop is around 6 mmHg in normal eyes and can be as much as 40 mmHg in glaucomatous eyes [1]. The intraocular pressure drops from 14 to 20 mm Hg to 9 mm Hg, that is the average episcleral venous pressure. It is something that to date has not been able to explain. As in other enigmas of the physiology of the body, the behavior of intraocular pressure has been tried to be explained based on theoretical mathematical models, constructed on concepts such as those described below [2].
Approximately 90% of aqueous outflow is through the trabecular meshwork. This flow is pressure-dependent, increasing as intraocular pressure increases. Aqueous humor flowing through the trabecular meshwork enters Schlemm’s canal and from there flows into the scleral, episcleral, and conjunctival venous systems (Figure 2). The trabecular meshwork consists of three layers: uveal, corneoscleral, and juxtacanalicular. Closest to the aqueous is the uveal meshwork. The corneoscleral meshwork lies deep to the uveal meshwork. This layer, like the uveal meshwork, does not offer significant resistance to aqueous outflow. The deepest layer of the trabecular meshwork is the juxtacanalicular tissue, the last layer that aqueous crosses before entering Schlemm’s canal. This juxtacanalicular tissue provides the most resistance to aqueous outflow.
Aqueous outflow occurs primarily through the posterior portion of the trabecular meshwork. With time, this posterior portion of the meshwork usually becomes pigmented, whereas the anterior meshwork usually remains relatively nonpigmented.
The uveal meshwork is dense and pigmented, giving a rough appearance to the trabecular meshwork and occasionally obscuring portions of the scleral spur. The uveal meshwork does not provide any resistance to aqueous outflow [3,4]. Most of the resistance in the aqueous outflow network occurs in the inner wall of Schlemm’s canal. Schlemm’s canal, which is a tube 190 to 350 μm in diameter at the base of the scleral sulcus, collects aqueous and drains it into the venous system. The aqueous veins are much too large to cause any appreciable resistance [5]. It had been shown that as IOP increases, the spaces in the trabecular meshwork expand. As the spaces open, the hydrodynamic resistance would decrease. Thus, if the corneoscleral meshwork were the principal site of flow resistance, one would expect the resistance to decrease rather than increase with an increase in IOP [6].
The effort of clinicians and researchers to try to explain an observable but so far inexplicable process is commendable. However, our discovery about the unsuspected ability of eukaryotic cells to dissociate the water molecule, through several molecules, the most important being melanin [7], offers the longawaited explanation of an important phenomenon to understand the dynamics of the aqueous humor.
The outflow of aqueous humor and water dissociation
Of the various hypotheses by which attempts have been made to explain the phenomenon of the notable decrease in intraocular pressure as it passes through the trabecular meshwork and Schlemm’s canal, none had raised the possibility that water, the main component of the aqueous humor, could dissociate into its gaseous elements as it passes through the trabecular meshwork.
It was unthinkable since it was considered that only the chlorophyll from the leaves of trees and plants could dissociate the water molecule. So, another 100 or two hundred years could have passed before understanding the mysterious and at the same time marvelous mechanism by which nature controls the outflow of 90% of the aqueous humor as it passes through the trabecular meshwork and Schlemm’s canal. Therefore, how is it possible that aqueous humor flows through the outflow network at an extremely low flow rate (2.0 μL/min), and this flow produces a surprisingly large pressure drop over a short flow distance (less than 1 mm)?
And apparently, the answer is that the melanin located inside the cells that make up the trabecular meshwork, transforms liquid water into its gaseous components, Hydrogen and Oxygen. As described in the following equation:
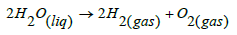
Which solves the puzzle, since no special anatomical structures are required to transport these gases to the outside of the eye, in this case to the aqueous veins. And Schlemm’s channel, the collecting channels, and the aqueous veins, transport the remaining aqueous humor, whose main component (water) was not dissociated.
And the dissociation of water by melanin, quite dependent on visible and invisible light, correlates very well with the wellknown circadian rhythm of intraocular pressure (IOP), that is: IOP tends to rise at night (when there is less light) reaching its maximum around 5 in the morning. And after dawn, intraocular pressure tends to drop (due to the presence of light), reaching its minimum level around 6 in the afternoon.
And just as the pattern of the anatomy of the human eye is repeated in all mammals, the presence of melanin is also clearly observed in the corneal scleral limbus, just outside the trabecular meshwork (Figure 3).
Conclusion
The mystery about the mechanism by which intraocular pressure decreases significantly when the aqueous humor crosses the trabecular meshwork, Schlemm’s canal, exiting through the collecting channels to reach the aqueous veins, has apparently been elucidated, which means an important advance in the fight against one of the three main causes of blindness in the world: glaucoma.
Acknowledgement
This work was supported by an unrestricted grant from Human Photosynthesis™ Research Centre, in Aguascalientes 20000, México.
References
- Johnson MC, Kamm RD (1983) The role of Schlemm's canal in aqueous outflow from the human eye. Invest Ophthalmol Vis Sci 24(3): 320-325.
- Schlichting H (1968) Boundary Layer Theory. New York, McGraw Hill, USA, p: 77.
- Moses RA (1979) Circumferential flow in Schlemm's canal. Am J Ophthalmol 88(3): 585-591.
- Wallace LM, Alward, Reid A Longmuir (2017) Anatomy of the Angle. In: Color Atlas of Gonioscopy. (2nd), American Academy of Ophthalmoscopy, USA.
- McEwen WK (1958) Application of Poiseuille's law to aqueous outflow. Arch Ophthalmol 60(2): 290-294.
- Johnstone MA, Grant WG (1973) Pressure-dependent changes in structures of the aqueous outflow system of human and monkey eyes. Am J Ophthalmol 75(3): 365-383.
- Herrera AS, Del CAEM, Md Ashraf G, Zamyatnin AA, Aliev G (2015) Beyond mitochondria, what would be the energy source of the cell? Cent Nerv Syst Agents Med Chem 15(1): 32-41.






























