Gene Regulation under Hypoxia: HIF1 Mediated Adaptation
Burcu Biterge Süt*
Department of Medical Biology, Niğde Ömer Halisdemir University, Turkey
Submission: February 08, 2018; Published: February 22, 2018
*Corresponding author: Burcu Biterge Süt, Niğde Ömer Halisdemir University, Faculty of Medicine, Medical Biology Department, Niğde, Turkey, Email: bbitergesut@ohu.edu.tr
How to cite this article: Burcu Biterge Süt. Gene Regulation under Hypoxia: HIF1 Mediated Adaptation. Anatomy Physiol Biochem Int J: 2018; 4(2): 555637. DOI: 10.19080/APBIJ.2018.04.555637.
Abstract
Oxygen is crucial for an organism to stay alive and the lack of sufficient oxygen is interpreted as an extreme stress condition. Adaptation to hypoxic conditions is a significant property of tumor cells which have evolved mechanisms to survive under this extreme stress condition. The master regulator of adaptation to low oxygen levels is a heterodimer protein called HIF1, which is differentially activated according to oxygen availability in the cellular environment.
Keywords: Hypoxia; Gene regulation; Cancer; HIF1; p53
Abbrevations: HIF-1: Hypoxia-Inducible Factor-1; TAD: Transactivation Domain; HAT: Histone Acetyl Transferase
Introduction
Mammalian cells require a certain level of oxygen in the environment in order to maintain homeostasis and to carry out aerobic respiration. However, in cancer and some other cardiovascular and pulmonary diseases, the cellular oxygen balance significantly changes in a way that pushes cells to survive under hypoxic conditions [1]. Survival under hypoxic conditions that arise from the insufficient delivery of oxygen via blood vessels to the rapidly growing tumor mass is a common feature of cancerous cells [2]. Cells that experience hypoxia develop adaptation to low-oxygen levels by activating cellular pathways related to cell survival, proliferation and metabolism. The ability of cancer cells to adapt to hypoxic conditions has been linked to tumor malignancy, resistance/unresponsiveness to treatment and poor prognosis [3].
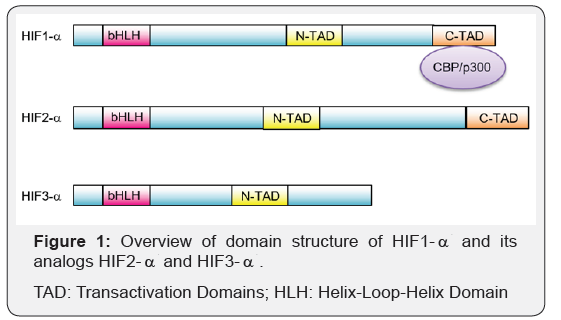
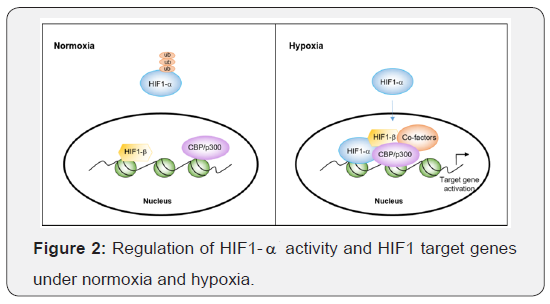
The master regulator of adaptation to hypoxia is a transcription factor called HIF-1 (Hypoxia-Inducible Factor-1) [4,5]. HIF1 is a heterodimer protein that is composed of HIF1- and HIF1- subunits. HIF1- and its analogs HIF2- and HIF3- have N-terminal and C-terminal transactivation domains (TADs) (Figure 1). C-TAD domain acts in regulation of gene activity by interacting with transcriptional regulators such as CBP/p300. On the other hand, N-TAD helps stabilize HIF1- and protects it from proteosomal degradation [6,7]. In contrast to the constitutively active HIF1- subunit, the expression of HIF1- subunit depends on the oxygen levels present in the environment. Under normal oxygen levels (normoxia), HIF1- undergoes proline hydroxylation by FIH (factor inhibiting HIF) enzyme, which then targets it for the ubiquitin ligase complex and leads to proteosomal degradation [8]. Under insufficient oxygen levels (hypoxia), FIH gets inactivated which in turn inhibits targeting HIF1- for proteosomal degradation. As HIF1- stabilizes and accumulates, it translocates to the nucleus where it joins with the subunit to form the HIF1 heterodimer [9].
Functional HIF1 heterodimer regulates more than a hundred genes by binding to the HRE (hypoxia responsive element) domains encompassing 5-[AG]CGTG-3 sequence [10], (Figure 2). Transcriptional coactivators such as CREBBP and CBP/p300 also contribute to the gene activation regulated by HIF1 under hypoxic conditions. HIF1 target genes are involved in several important cellular processes such as energy metabolism, glucose metabolism, angiogenesis, apoptosis and proliferation (Table 1). Therefore, HIF1 mediated regulation is considered a key regulator of embryonic vascularization, tumor angiogenesis and the pathophysiology of ischemic diseases. The transcriptional regulation of p53 which plays a significant role in cellular adaptation to genotoxic stress is carried out in a similar manner to that of HIF1-‘s. p53 is an important tumor suppressor protein that is involved in DNA damage, cell cycle control, apoptosis and senescence. Although it is expressed at low levels under normal conditions, upon DNA damage it gets phosphorylated and stabilized, which then provides target gene regulation [11, 12].

In addition, under hypoxic conditions p53 regulates a different set of target genes using a different mechanism than the DNA damage pathway. Previous studies indicate that there is a cross-regulation at the transcriptional level between HIF1 and p53 which are two important players in developing cellular adaptation to various stress conditions [13]. This crossregulation can either be activating or repressing depending on several other factors. Furthermore, under hypoxic conditions HIF1 and p53 compete to bind p300 which is required for the transcriptional activation of them both [14]. The fact that p300 and PCAF (p300/CBP Associated Factor) which is the other transcriptional co-activator that participates in this regulation possess histone acetyltransferase (HAT) property hints towards epigenetic regulation in developing cellular adaptation to hypoxia.
Conclusion
Cellular adaptation to low oxygen levels involves a complex regulatory mechanism that allows the cells to maintain their homeostasis and survive. The interplay between HIF1, the master regulator of the response to hypoxia, and one of the most important tumor suppressors p53 is very important for cancer cells to gain the ability to thrive under hypoxic conditions. This interaction between HIF1 and p53 also points at the significance of transcriptional co-activators such as CBP/p300, both at the level of target gene regulation and their roles in modulating chromatin as histone acety transferases. Further studies towards HIF1, p53 and the epigenetic regulation between them will enable researchers to have a better understanding of cancer cell survival under hypoxia.
Conflict of Interest
The author declares no conflict of interest.
References
- Semenza GL (2000) HIF-1 and human disease: one highly involved factor. Genes Dev 14(16): 1983-1991.
- Powis G, Kirkpatrick L (2004) Hypoxia inducible factor-1 alpha as a cancer drug target. Mol Cancer Ther 3(5): 647-654.
- Semenza GL (2003) Targeting HIF-1 for cancer therapy. Nat Rev Cancer 3(10): 721-732.
- Semenza GL (2001) HIF-1, O(2), and the 3 PHDs: how animal cells signal hypoxia to the nucleus. Cell 107(1): 1-3.
- Pugh CW, Ratcliffe PJ (2003) Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med 9(6): 677-684.
- Masoud GN, Li W (2015) HIF-1α pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B 5(5): 378-389.
- Yamashita K, Discher DJ, Hu J, Bishopric NH, Webster KA (2001) Molecular regulation of the endothelin-1 gene by hypoxia. Contributions of hypoxia-inducible factor-1, activator protein-1, GATA- 2 and p300/CBP. J BiolChem 276(16): 12645-12653.
- Ivan M, Kondo K, Yang H, Kim W, Valiando J, et al. (2001) HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292(5516): 464-468.
- Chen C, Lou T (2017) Hypoxia inducible factors in hepatocellular carcinoma. Oncotarget 8(28): 46691-46703.
- Ratcliffe PJ, O’Rourke JF, Maxwell PH, Pugh CW (1998) Oxygen sensing, hypoxia inducible factor-1 and the regulation of mammalian gene expression. J Exp Biol 201(Pt 8): 1153-1162.
- Wu X, Bayle JH, Olson D, Levine AJ (1993) The p53-mdm-2 autoregulatory feedback loop. Genes Dev 7(7A): 1126-1132.
- Prives C (1998) Signaling to p53: breaking the MDM2-p53 circuit. Cell 95(1): 5-8.
- Obacz J, Pastorekova S, Vojtesek B, Hrstka R (2013) Cross-talk between HIF and p53 as mediators of molecular responses to physiological and genotoxic stresses. Mol Cancer 12(1): 93.
- Freedman SJ, Sun Z-YJ, Poy F, Kung AL, Livingston DM, et al. (2002) Structural basis for recruitment of CBP/p300 by hypoxia-inducible factor-1 alpha. Proc Natl Acad Sci USA 99(8): 5367-5372.






























