Alleviation of Acute Poisoning of Organophosphates in Humans
Vivek Kumar Gupta1 and Bechan Sharma2*
1Department of Biochemistry, University of Allahabad, India
2Department of Biochemistry, University of Allahabad, India
Submission: September 25, 2016; Published: October 12, 2016
*Corresponding author: Bechan Sharma, Department of Biochemistry, University of Allahabad, Allahabad-211002, India, Tel:+91-9415715639; Email: sharmabi@yahoo.com
How to cite this article: Vivek Kumar G, Bechan S. Alleviation of Acute Poisoning of Organophosphates in Humans. Anatomy Physiol Biochem Int J. 2016; 1(2) : 555558. DOI: 10.19080/APBIJ.2016.01.555558
Abstract
Organophosphates (OPs) are used as pesticides and developed as warfare nerve agents. Exposure to an organophosphate could be lethal resulting in death due to respiratory failure. The mechanism of organophosphate poisoning includes inhibition of the acetyl cholinesterase (AChE) via phosphorylation of the hydroxyl group of serine present at the active site of the enzyme. The inhibition of acetyl cholinesterase (AChE) results in the accumulation of acetylcholine (ACh) at cholinergic receptor sites, producing continuous stimulation throughout the nervous systems. Several therapeutic agents have been developed and used in the treatment of poisoning with OPs. For example, pyridiniumoximes have been developed as therapeutic agents for the treatment of poisoning by OPs. The mode of action of pyridiniumoximes is by the reactivation of inhibited acetyl cholinesterase. However, the universal broad spectrum oximes capable of protecting against all known OPs, is still have to be investigated. Presently, a combination of an antimuscarinic agent, e.g. atropine, an AChE reactivators i.e. oximes and diazepam are used for the treatment of organophosphate poisoning in humans. In spite of enormous efforts devoted to the development of new AChEreactivators as antidotes against poisoning with organophosphates, only four compounds so far have been found their applications in human medicine. This article presents an updated account of the available reports concerning the treatment of OP poisoning and its alleviation.
Keywords: Pesticides; Organophosphate Poisoning; Antidote; Acetyl cholinesterase; Cholinergic; Alleviation
Abbreviations: OP: Organophosphate; AChE: Acetylcholinesterase; ChE: Cholinesterases; Ach: acetylcholin
Introduction
Acetylcholinesterase (AChE) (EC 3.1.1.7) is the primary cholinesterase belongs to carboxylesterase family . It is an acetylhydrolase, found in many types of conducting tissues. AChE is also found on the red blood cell membranes and blood plasma (EC 3.1.1.8, ChE) [1]. The function of AChE is the termination of ACh at the junctions of the various cholinergic nerve endings with their post-synaptic sites which catalyzes the breakdown of acetylcholine that function as neurotransmitters with very high catalytic activity. The turn over number for AChE has been found to be about 25000 molecules of acetylcholine (ACh) hydrolysed per second [2]. The AChE activity is higher in motor neurons than in sensory neurons [3,4]. AChE exists in multiple molecular forms with different oligomeric assembly but having the same catalytic activities. The enzyme has been reported to be membrane bound [5-7]. The active site of AChE has two sub sites - anionic site and esteraticsubsite.
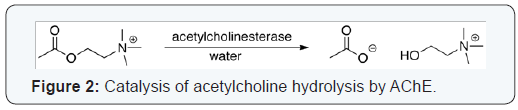
The esteraticsubsite contains the catalytic triad of three amino acids: serine 200, histidine 440 and glutamate 327 similar to the triad in other serine proteases except that the glutamate is the third member rather than aspartate, where acetylcholine is hydrolyzed to acetate and choline [8]. The hydrolysis reaction of the carboxyl ester forms an acyl-enzyme and free choline. Then, the acyl-enzyme undergoes nucleophilic attack by a water molecule, assisted by the histidine 440 group, liberating acetic acid and regenerating the free enzyme [9,10]. The mechanism of action of AChE has been elucidated in (Figure 1). The anionic sub site accommodates the positive quaternary amine of acetylcholine and other cationic substrates and inhibitors. The cationic substrates are not bound by interaction of 14 aromatic amino residues [11], which are highly conserved across different species [12]. Among these aromatic amino acids the substitution of tryptophan 84 with alanineresults in a 3000-fold decreased reactivity [13]. During neurotransmission, ACh is released from presynaptic neuron into synaptic cleft and binds to ACh receptors on the post-synaptic membrane, relaying the signal. AChE, also located on the post-synaptic membrane, terminates the signal transmission by hydrolyzingACh. The liberated choline is taken up again by the pre-synaptic neuron and ACh is synthesized by combining with acetyl-CoA through the action of choline acetyltransferase [14] (Figure 2).


Organophosphates (OPs), the esters of phosphoric acid, are a class of irreversible AChE inhibitors. The cleavage of OP by AChE leaves a phosphoryl group in the esteratic site, which is slow to be hydrolyzed and can bound covalently. Carbamates, esters of N-methyl carbamic acid, are reversible inhibitors of AChE that hydrolyze in hours and occupy the esteratic site for short periods of time (Figure 3). Presently, a combination of AChE reactivators such as atropine and diazepam are used for the treatment of OP poisoning. The drugs donepezil, galantamine, and rivastigmine used in alzheimer disease are inhibitors of AChE [9,15]. It has also been reported that some phytochemicals such as tetrahydrocannabinol, the active ingredient of cannabis, is a competitive inhibitor of AChE [16]. This article presents an updated account of the reports available concerning the alleviation of OP poisoning by some antidotes including atropine and oximes.
Interaction of cholinesterases with organophosphates
The physiological role of AChE in blood is not understood, but it was proposed that ChE may have roles in neurotransmission and involved in other nervous system functions and in neurodegenerative disorders [17]. In the presence of OPs, AChE becomes progressively inhibited and is not further capable of hydrolyzing ACh [18]. Consequently, ACh accumulates at cholinergic receptor sites and produces excessive stimulation of cholinergic receptors throughout the nervous systems. Both substrate and inhibitors react covalently with the esterase in essentially the same manner, because acetylation of the serine residue at AChE catalytic site is analogous to phosphorylation. Inhibited enzyme can be spontaneously reactivated at different rates depending on the inhibitor. The variations in the acute toxicity of OP are the result of their different chemical structures and rates of spontaneous reactivation and aging. The aging has the major clinical importance and an imperative problem in the treatment of pesticide poisoning because aged form of phosphorylated AChE is resistant to both spontaneous and oxime-induced reactivation. Hence, recovery of inactivated AChE function depends on relatively slow resynthesis of AChE during aging thereby exerting higher level of toxicity as compared to those at younger age.
Clinical presentation of OP poisoning
According to World Health Organization (WHO), in cases of intoxication the signs and symptoms of acute poisoning with OPs are predictable from their levels of AChE activity [19]. These clinical features include sweating, lacrimation, rhinorrhea, and abdominal cramps, salivation, respiratory difficulties, dyspnea, cough, wheezing, fasciculations, bradycardia, change in ECG, cyanosis, anorexia, nausea, vomiting, diarrhea, involuntary urination and defecation, accompanied by dizziness, tremulousness, confusion, ataxia, headache, tremors, constriction sensation in the chest, twitching of facial muscles and tongue, and fatigability finally into death. It has been reported that even after survival of the patient with OP poisoning, there would be mood swings, personality changes, aggressive events and psychotic episodes [20,21]. Diagnosis is relatively based on medical history, exposure circumstances, clinical presentation, and laboratory tests. Erythrocyte AChE is identical to the enzyme present in the target synapses and its levels are assumed to reflect the effects of OPs in target organs. Thus, erythrocyte AChE may be considered as a biomarker of neurotoxicity. Due to pharmacokinetic reasons, it is difficult to know exactly, how closely the AChE inhibition in erythrocytes reflects to that in the nervous system since access to blood is always easier than brain. Thus, erythrocytes AChE inhibition may reflect altogether a different message from that in brain [18].
Treatment of acute poisoning with Organophosphorus pesticides
Treatment of OP pesticide poisoning should begin with decontamination and care must be taken not to contaminate others.
Atropine
Atropine acts through blocking the effects of excess concentrations of acetylcholine at cholinergic synapses following OP inhibition of AChE. It has been reported that atropine may prevent development of convulsions and brain damage induced by certain OP [22]. The trial dose of atropine is 0.05 mg/kg intravenously, should be given slowly over 3 min, and then repeated every 5–10 min. In symptomatic children, intravenous dose of 0.015–0.05 mg/kg atropine should be administered at interval of every 15 min. Atropine may then be repeated at 15– 30 min intervals until the patient is atropinized (dilated pupils, dry skin, and skin flushing) which should be maintained during further treatment.
Diazepam
Benzodiazepines are central nervous system (CNS) depressants, anxiolytics (antipanic or antianxiety agent) and muscle relaxants. Marrs [23] in has reported that benzodiazepines, including diazepam, alter GABA binding in an allosteric fashion. The recommended dose of diazepam in cases of OP poisoning is 5–10mg intravenously in the absence of convulsions and 10–20mg intravenously in cases with convulsions [22].
Oximes
The antidotal potency of pyridiniumoximes is primarily attributed to their ability to reactivate phosphorylated cholinesterases. Reactivation proceeds through the formation of intermediate Michaelis-Menten complex leading to the formation of stable phosphoryl residue bound to the hydroxyl group of serine present at active site of AChE. The rate of reactivation depends on structure of phosphoryl moiety bound to the enzyme, source of the enzyme, rate of post inhibitory dealkylation and concentration of oxime [24,25]. Pyridiniumoximes are effective in the peripheral nervous system, but also have a penetration across the blood–brain barrier [26] and therefore enable passage of higher oxime concentrations into brain [27]. Pralidoxime is not sufficiently effective in the treatment of OP pesticide poisoning [28]. The inadequate initial treatment with oximes may not be sufficiently effective in OP poisoning because oximes are rapidly cleared from the body. Among the many classes of oximes investigated with clinical application can be divided in following groups: monopyridinium (PAM-2, pralidoxime), bispyridiniumoximes (TMB-4, trimedoxime), obidoxime (LuH-6, Toxogonin) and asoxime (HI-6).
Pralidoxime (PAM-2)
Sidell and Groff (1971) have shown that the pralidoxime administered to human at a dose of 10 mg/kg by intramuscular route, produced a plasma concentration of >4 mg/L within 5–10 min and maintained levels above this threshold for an hour [29]. The PAM-2 iodide was given in combination with atropine and diazepam, in the treatment of the victims of Tokyo sarin attack victims in 1995 [30]. However, PAM-2 should not be recommended against poisoning with warfare nerve agents due to its lack of efficacy [31].
Obidoxime
Obidoxime when administered to humans by intramuscular route, it produced a plasma concentration >4 mg/L, from 5 min after injection to 3 h [32]. Following high doses of obidoxime in severely OP poisoned patients; occasional hepatotoxic effects have been observed including increased serum transaminases and jaundice [33].
Asoxime (HI-6)
Asoxime is considered to be a very promising bispyridiniumoxime in treatment following exposure to most nerve agents. Studies showed that Asoxime dosed by intramuscular route reached plasma concentrations >4 mg/L in 4–6 min [34]. According to Jovanovi´c et al. [35] asoxime did not show any adverse effect on humans. The only disadvantage of asoxime compared to other available oximes is its lack of stability in aqueous solutions. Asoxime was considered to be an effective antidote in treatment of patients poisoned with OP insecticides [34].
Conclusion
The management of acute Organophosphate pesticide poisoning in humans includes general (decontamination and supportive measures) and specific treatment with atropine, oximes (pralidoxime, trimedoxime, obidoxime, and asoxime) and diazepam. Since the introduction of the antidotes in treating the patients poisoned with OPs, there is still no agreement on how these substances should be given for the best result following treatment. While the use of atropine and diazepam in humans have been widely accepted throughout the world., Pyridiniumoximes were successful in the treatment of most cases of OP poisoning, when given with atropine and diazepam. However, some reports indicate that treatment with pralidoxime was not sufficiently beneficial. These problems of effectiveness of oxime treatment may be solved in randomized clinical trial(s) comparing the WHO-recommended regimen with a placebo to assess the value of pralidoxime, and other oximes (obidoxime, trimedoxime, and Asoxime) as well, in acute poisoning with OPs.
Acknowledgement
Vivek Kumar Gupta is grateful to the University Grant Commission, New Delhi, for providing financial assistance in the form of a Research Fellowship.
References
- CF Bartels, T Zelinski, O Lockridge (1993) Mutation at codon 322 in the human acetylcholinesterase (ACHE) gene accounts for YT blood group polymorphism. Am J Hum Genet 52(5): 928-936.
- P Taylor, Z Radić (1994) The cholinesterases: from genes to proteins. Annual Review of Pharmacology and Toxicology 34: 281-320.
- GB Koelle (1954) The histo chemical localization of cholinesterases in the central nervous system of the rat. Journal of Comparative Anatomy 100(1): 211-235.
- LW Chacko, JA Cerf (1960) Histo chemical localization of cholinesterase in the amphibian spinal cord and alterations following ventral root section. Journal of Anatomy 94: 74-81.
- VK Gupta, R Pal, NJ Siddiqi, B Sharma (2015) Acetylcholinesterase from human erythrocytes as a surrogate biomarker of lead induced neurotoxicity. Enzyme Res.
- VK Gupta, A Kumar, NJ Siddiqi, B Sharma (2015) Rat brain acetyl cholinesterase as a biomarker of cadmium induced neurotoxicity. Open Access J of Tox 1(1): 555553.
- VK Gupta, APathak, NJ Siddiqi, B Sharma (2016) Carbofuran modulating functions of acetylcholinesterase from rat brain in vitro. Advances in Biology.
- A Tripathi (2008) Acetylcholinsterase: A versatile enzyme of nervous system. Annals of Neurosciences 15(4): 106-111.
- LM Pohanka (2011) Cholinesterases, a target of pharmacology and toxicology. Biomedical Papers Olomouc 155(3): 219-229.
- M Pohanka (2012) Alpha7 nicotinic acetylcholine receptor is a target in pharmacology and toxicology. International Journal of Molecular Sciences 13(12): 2219-2238.
- N Ariel, A Ordentlich, D Barak, T Bino, B Velan, et al. (1998) The aromatic patch of three proximal residues in the human acetylcholinesterase active centre allows for versatile interaction modes with inhibitors. Biochem J 335(1): 95-102.
- A Ordentlich, D Barak, C Kronman, Y Flashner, M Leitner, et al. (1993) Dissection of the human acetylcholinesterase active center determinants of substrate specificity. Identification of residues constituting the anionic site, the hydrophobic site, and the acyl pocket. J Biol Chem 268(23): 17083-17095.
- V Tougu (2001) Acetylcholinesterase: mechanism of catalysis and inhibition. Current Medicinal Chemistry Central Nervous System Agents 1(2): 155-170.
- VP Whittaker (1990) The contribution of drugs and toxins to understanding of cholinergic function. Trends in Physiological Sciences 11(1): 8-13.
- M Pohanka (2011) Alzheimer´s disease and related neurodegenerative disorders: implication and counteracting of melatonin. Journal of Applied Biomedicine 9(4): 185-196.
- LM Eubanks, CJ Rogers, AE Beuscher, GF Koob, AJ Olson et al. (2006) A molecular link between the active component of marijuana and Alzheimer’s disease pathology. Mol Pharm 3(6): 773-777.
- S Darvesh, DA Hopkins, C Geula (2003) Neurobiology of butyrylcholinesterase. Nat Rev Neurosci 4(2): 131-138.
- M Jokanovi´, M Maksimovi´ (1997) Abnormal cholinesterase activity: understanding and interpretation. Eur J ClinChemClinBiochem 35(1): 11-16.
- LM Pohanka (2011) Cholinesterases, a target of pharmacology and toxicology. Biomedical Papers Olomouc 155(3): 219-229.
- TC Marrs, JA Vale (2006) Management of organophosphorus pesticide poisoning. In: RC Gupta (Edn.), Toxicology of Organophosphorus and Carbamate Compounds. Elsevier Academic Press, Amsterdam, Holland, pp 715-733.
- AG Karchmar (2007) Anticholinesterases and war gases. In: AG Karchmar (Edn.), Exploring the Vertebrate Central Cholinergic Nervous System. Springer pp 237-310.
- B Antonijevi´, MP Stojiljkovi´ (2007) Unequal efficacy of pyridiniumoximes in acute organophosphorus poisoning. Clin Med Res 5(1): 71-78.
- TC Marrs (2004) Diazepam. Antidotes for Poisoning by Organophosphorus Pesticides. International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland.
- Y Ashani, AK Bhattacharjee, H Leader, A Saxena, BP Doctor (2003) Inhibition of cholinesterases with cationic phosphonyloximes highlights distinctive properties of the charged pyridine groups of quaternary oximereactivators. BiochemPharmacol 66(2): 191-202.
- F Worek, P Eyer, N Aurbek, L Szinicz, H Thiermann (2007) Recent advances in evaluation of oxime efficacy in nerve agent poisoning by in vitro analysis. Toxicol Appl Pharmacol 219(2-3): 226-234.
- VGrange-Messent, C Bouchaud, M Jamme, G Lallement, A Foguin et al. (1999) Seizure-related opening of the blood–brain barrier produced by the anticholinesterase compound, soman: new ultrastructural observations. Cell MolBiol 45(1): 1-14.
- S Shrot, G Markel, T Dushnitsky, A Krivoy (2009) The possible use of oximes as antidotal therapy in organophosphate-induced brain damage. Neurotoxicology 30(2): 167-173.
- MA Cherian, C Roshini, JV Peter, AM Cherian (2005) Oximes in organophosphorus poisoning. Indian J Crit Care Med 9(3): 155-163.
- FR Sidell, WA Groff (1971) Intramuscular and intravenous administration of small doses of 2-pyridinium aldoximemethochloride to man. J Pharm Sci 60(8): 1224-1228.
- MP Stojiljkovi´, M Jokanovi´ (2005) AUM Shinrikyo and terrorist use of nerve agents in Japan. In: A Monov, C Dishovsky (Edn.), Medical Aspects of Chemical and Biological Terrorism: Chemical Terrorism and Traumatism. Publ
- J Kassa (2005) The role of oximes in the antidotal treatment of chemical casualties exposed to nerve agents. In: A Monov, C Dishovsky (Edn.), Medical Aspects of Chemical and Biological Terrorism: Chemical Terrorism and Traumatism. Publishing House of the Union of Scientists in Bulgaria, Europe, pp 193-208.
- FR Sidell, WA Groff (1970) Toxogonin: blood levels and side effects after intramuscular administration in man. J Pharm Sci 59(6): 793-797.
- P Eyer (2003) The role of oximes in the management of organophosphorus pesticide poisoning. Toxicol Rev 22(3): 165-190.
- R Kuˇsi, D Jovanovi , S Ran–delovi, D Joksovi, V Todorovi, et al. (1991) HI-6 in man: efficacy of the oxime in poisoning by organophosphorus insecticides. Hum ExpToxicol 10(2): 113-118.
- D Jovanovi´, M Maksimovi´, D Joksovi´, V Kovaˇcevi´ (1990) Oral forms of the oxime HI-6: a study of pharmacokinetics and tolerance after administration to healthy volunteers. Vet Hum Toxicol 32(5): 419-421.






























